-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 by Daniela Vrabelova Ackley, DVM DACVIM
by Daniela Vrabelova Ackley, DVM DACVIM
www.angell.org/internalmedicine
internalmedicine@angell.org
781-902-8400
MSPCA-Angell West, Waltham
Glomerular diseases (GD), characterized by proteinuria, are a leading cause of renal morbidity and failure in dogs and have seemingly poor outcomes when treated with current therapies. The International Renal Interest Society (IRIS) appointed a group of experts in veterinary nephrology to develop practical clinical guidelines to improve outcomes in patients with GD.
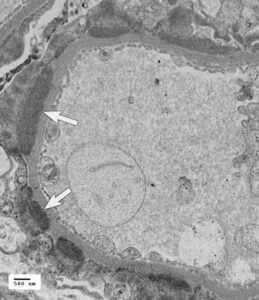
Fig 1. Transmission electron micrograph (15,000x) of a canine glomerular capillary loop demonstrating deposition of subepithelial deposits (arrows) consistent with immune complexes (Courtesy of Dr. Lees and Clubb, Texas Veterinary Renal Pathology Service).
The consensus stressed the importance of renal biopsy in animals with glomerular lesions that have not progressed to an end stage, in order to achieve a definitive diagnosis, prognosis and offer appropriate treatment options based on the type, severity, and biologic behavior of the underlying injury. Kidney biopsy became abandoned as a diagnostic tool in veterinary medicine during most of the 20th century given the inconsistent pathology reports providing information of little clinical use and high rate of complications. Only limited information can be obtained by light microscopy used in the past therefore the World Small Animal Veterinary Association (WSAVA) launched an ambitious 6-year Renal Standardization Project (RSP). Main goals of this project were to characterize canine GD employing advanced techniques, such as transmission electron and immunofluorescent microscopy, and to relate the pathologic findings to patient presentations and outcomes (Fig 1. and Fig 2.).
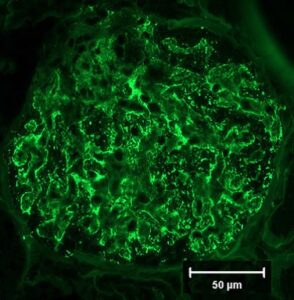
Fig 2. Immunofluorescent image of a canine glomerulus (40x) immunostained for IgG demonstrating extensive capillary wall staining and segmental mesangial staining documenting immune deposits and predicting an immune-driven pathogenesis to the glomerular injury (Courtesy of Dr. Lees, Texas Veterinary Renal Pathology Service).
Furthermore, this project offers a more consistent classification of GD and correlates pathologic diagnoses with clinical data to help in predicting treatment response. The WSAVA RSP represents a global diagnostic resource and database for general and specialty veterinarians throughout the world mirroring the level of sophistication in human nephrology.
As dogs with GD may present differently and require a different approach than those with whole nephron or tubular disease, the IRIS formulated the following guidelines that were implemented by many internists and can be helpful for primary care veterinarians.
Proteinuria is considered a hallmark of glomerular lesions. The first and crucial step is to determine the renal origin of proteinuria along with the magnitude and persistence.
Diagnostic recommendations for all cases with suspected GD:
- Comprehensive history including family, travel, drug exposure and diet
- Physical examination including retinal and rectal examination and blood pressure (at least 2 readings on different days)
- CBC, complete biochemistry, urinalysis and urine sediment evaluation
- Urine culture (if microscopic pyuria, hematuria, bacteriuria, USG < 1.025, azotemia, or hyperadrenocorticism, diabetes, etc. with possible occult infection)
- Urine protein/creatinine ratio (UPC), at least 2 readings
- In endemic areas infectious disease testing (Lyme disease, heartworm, Ehrlichiosis, Leishmaniosis)
The presentations of various glomerular diseases are diverse and range over a wide spectrum of severity. What is reasonable to recommend for investigation of proteinuria in an otherwise healthy dog differs from what should be done for a dog with proteinuria, azotemia, hypoalbuminemia, hypertension, and edemas. To facilitate matching recommendations, experts group proteinuric dogs into three tiers of increasing disease severity:
Tier I – persistent renal proteinuria
- Tier I-A – subclinical
- Tier I-B – with hypertension
Tier II – renal proteinuria and hypoalbuminemia
- Tier II-A – with or without associated complications (edemas, thromboembolic events)
- Tier II-B – same as Tier II-A with hypertension
Tier III – renal proteinuria and renal azotemia
- Tier III-A –only proteinuria and azotemia
- Tier III-B – proteinuria, azotemia and hypertension
- Tier III-C – proteinuria, azotemia, hypoalbuminemia, with or without hypertension
Repeating UPC determinations
Repeated UPC determinations are needed in all the patients, regardless of the magnitude of proteinuria. In cases with mild proteinuria (UPC < 2), repeated UPC measurements are recommended to verify persistence of proteinuria and establish the likelihood of GD. In dogs with a large magnitude of proteinuria repeated UPC measurements are less needed to verify the presence of proteinuria but will help to reliably estimate the UPC. The day-to-day variability is high in patients with marked proteinuria, making it necessary to average the values obtained on several days.
Additional diagnostics are recommended for dogs with suspected GD associated with high magnitude (UPC > 3.5) or progressive proteinuria, hypertension, hypoalbuminemia, and/or azotemia:
- Complete abdominal ultrasound
- Thoracic radiographs (search for infiltration, effusion)
- More comprehensive evaluation for infectious diseases
- If hypertension present look for extrarenal causes (hyperadrenocorticism, hyperaldosteronism, pheochromocytoma, drug side effect, fluid and salt overload, etc.), consider echocardiography to look for concentric left ventricular hypertrophy
- If hypoalbuminemia, azotemia, or both, rule out neoplasia (imaging, lymph node or bone marrow aspirate), rule out other causes of hypoalbuminemia (malnutrition, liver disease, gastrointestinal loss)
- Renal biopsy is recommended, especially if the proteinuria is substantial (UPC > 3.5), unresponsive to treatment, or if immunosuppressive therapy is contemplated (except for end-stage patients)
- Other considerations: antithrombin testing, thromboelastography, Bence Jones protein or SDS-Page test to differentiate glomerular form tubular proteinuria, and DNA banking for studies of predisposed breeds
Renal biopsy
Experienced personnel at all stages of procuring, preparing, and interpreting the renal biopsy, not only by light microscopy but also electron microscopy and immunofluorescence, is paramount to get useful information. Light microscopy alone usually cannot reliably determine whether immune-mediated GD exists, and decision-making concerning the use of immunosuppressive protocols is hampered. It is essential that renal biopsies of dogs with GD contain adequate samples of cortex and are properly processed using suitable fixatives, and submitted to a pathology laboratory with experience in advanced renal histopathology. The center must be contacted before biopsy samples are obtained to provide renal biopsy kits and instructions. Currently available centers providing such services include: International Veterinary Renal Pathology Service, a joint collaboration of Texas A&M and The Ohio State University (contact: Dr. Rachel Cianciolo; Rachel.cianciolo@cvm.osu.edu).
Consensus recommendations for standard therapy of glomerular disease in dogs
Standard therapy forms the basic or routine care of dogs with GD and is recommended for use in all affected animals regardless of the inciting cause.
The magnitude of proteinuria, assessed by serial measurements of the UPC, should be used to make decisions about therapeutic interventions. There is evidence that dogs exhibit more adverse outcomes if the UPC exceeds 2.0. Intervention should be considered whenever renal proteinuria is causing the UPC to persistently exceed 0.5 in a dog with glomerular disease (primary or secondary). A reduction in the UPC to < 0.5 or a reduction by 50% is considered a therapeutic success.
| Category/purpose | Drug | Initial Dose |
| Angiotensin converting enzyme inhibitor | Benazapril | 0.5 mg/kg po q24h |
| Enalapril | 0.5 mg/kg po q24h | |
| Ramipril | 0.125 mg/kg po q 24h | |
| Imidapril | 0.25 mg/kg po q24h | |
| Angiotensin receptor blocker | Telmisartan | 1 mg/kg po q24h |
| Losartan | 0.125-0.5 mg/kg po q24h* | |
| Aldosterone receptor blocker | Spironolactone | 1-2 mg/kg po q12h |
| Dietary polyunsaturated fatty acids | n-6/n-3 ratio 5:1 | |
| Antithrombotics | Aspirin | 0.5-5 mg/kg q24h |
| Clopidogrel | 1.1 mg/kg q24h | |
| Antihypertensive agents | Amlodipine | 0.1-0.75 mg/kg q24h |
| Atenolol | 0.25-1 mg/kg q12h | |
| Prazosin | 0.5-2 mg/kg q8-12h | |
| Phenoxybenzamine | 0.25 mg/kg q8-12h | |
| Hydralazine | 0.5 mg/kg q12 | |
| Acepromazine | 0.5-2 mg/kg q8h | |
| Diuretics | Hydrochlorothiazide | 2-4 mg/kg q12-24h |