-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Becca Reader, DVM, DACVAA
By Becca Reader, DVM, DACVAA![]()
angell.org/anesthesia
anesthesia@angell.org
617-541-5048
April 2023
x
x
x
Gabapentin Prescribing Practices
Gabapentin is an anti-epileptic and analgesic drug originally intended to be a centrally acting gamma-aminobutyric acid (GABA)-receptor agonist.1 Gabapentin is currently labeled by the FDA for use in humans as an anticonvulsant and for treating pain associated with spinal cord injuries, fibromyalgia, post-herpetic neuralgia, and neuropathic pain associated with diabetes.2 Despite this narrow indication for use, gabapentin is one of the top 10 most frequently prescribed medications in the United States, with over 80% of prescriptions for extra-label use.3,4
Veterinary use of gabapentin has also increased dramatically over the past several years, likely due to the desire to have an oral analgesic alternative to non-steroidal anti-inflammatory drugs (NSAIDs). A recent survey of veterinarians found that 69% of respondents prescribe gabapentin daily or weekly, most commonly for acute and chronic pain.5 Despite this popularity, gabapentin has a similarly narrow indication for use in veterinary medicine, and current prescribing practices warrant further scrutiny.
Mechanism of Action
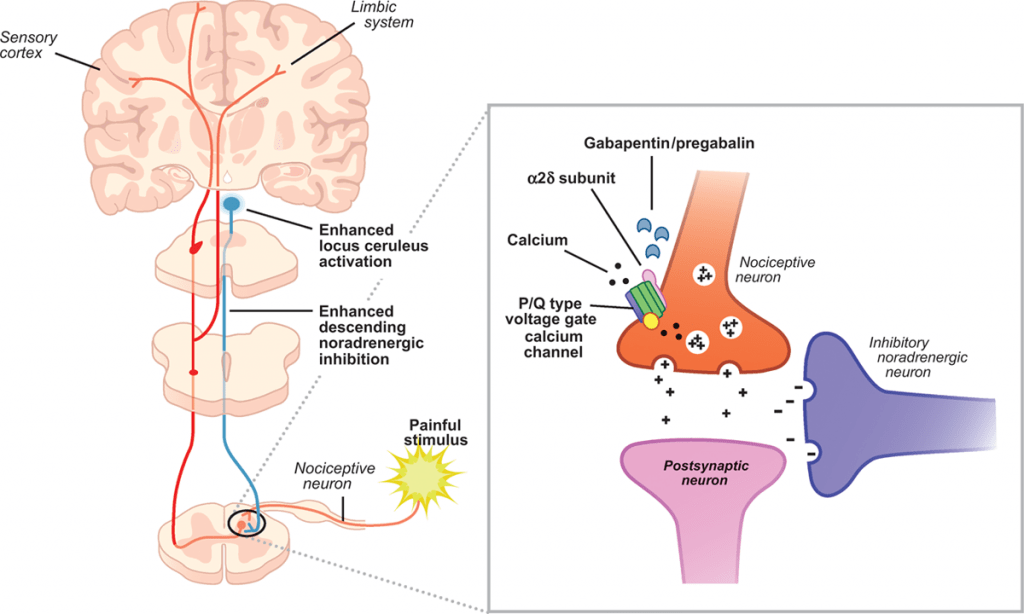
Understanding the mechanism of action of gabapentin is critical when evaluating the role that gabapentin may have as an analgesic for veterinary patients. As mentioned, gabapentin was initially intended to be a centrally acting agonist at the GABA receptor. However, while structurally similar to the GABA molecule, gabapentin does not bind to the GABA receptor or influence the action or metabolism of the GABA molecule.1 Instead, gabapentin is believed to bind to receptors on calcium channels on the presynaptic neurons in the central nervous system. The binding of gabapentin to these receptors blocks the influx of calcium into the presynaptic nerve terminal, decreasing the release of excitatory neurotransmitters.1
Mammalian pain transmission involves the conversion of a noxious stimulus to an electrical signal, which is then transmitted by peripheral sensory fibers to the dorsal horn of the spinal cord.6 Pain signals are either amplified or suppressed by endogenous neurotransmitters or analgesic drugs in the dorsal horn and then progress to the brain, where the signal is consciously perceived.6 Left untreated amplification of pain signals in the dorsal horn can lead to maladaptive or chronic pain states.6
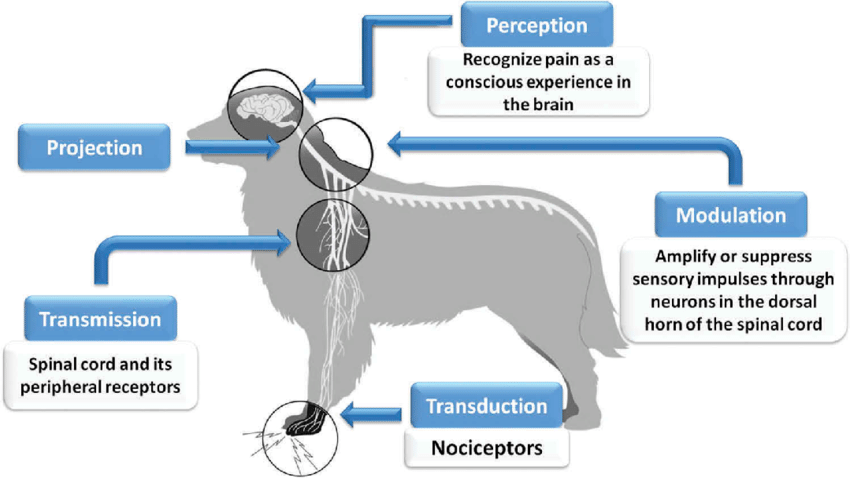
Analgesic properties of gabapentin are believed to be due to the blockade of calcium channels on presynaptic nerve terminals in the dorsal horn of the spinal cord, which decreases the release of excitatory neurotransmitters (e.g., substance P, glutamate, glycine) that would otherwise amplify pain signals. Accordingly, in most circumstances, gabapentin is best thought of as a modulator of pain; administration may decrease the transmission of pain signals but is unlikely to stop pain signals completely.
Targeted Use of Gabapentin
One of the most commonly cited uses of gabapentin in veterinary medicine is for treating acute post-operative pain.5 Considering the mechanism of action of gabapentin and its impact on pain signaling, it is unlikely that gabapentin will be an effective analgesic in this context. Inflammation is the most common component of acute post-operative pain, and while gabapentin modulates pain signals from the periphery, it does not treat inflammation directly. As a result, gabapentin may reduce pain signaling associated with inflammatory pain, but it will not address the source of pain directly.
Gabapentin administration is better applied in treating neuropathic pain and complex pain states requiring a multi-modal approach to achieve sufficient analgesia. A primary central or peripheral nervous system lesion, such as intervertebral disc herniation, plexus avulsions, and nerve root impingement, causes neuropathic pain.6,7 Imbalances between excitatory and inhibitory pain signaling contribute to the development of neuropathic pain,7 and administration of gabapentin may help reduce the amount of excitatory pain signals generated and subsequently transmitted to the cerebral cortex.
Gabapentin may also be added to an analgesic regimen to manage heightened pain states if first-line analgesics are insufficient. Examples of heightened pain states include polytrauma, pathologic fractures, cancer pain, thrombosis, or conditions associated with extensive inflammation, such as peritonitis and fasciitis. Again, gabapentin’s inhibition of presynaptic calcium channels reduces excitatory pain signaling, improving analgesia. Gabapentin may also act synergistically in combination with other analgesics, reducing required doses and minimizing adverse effects (e.g., dysphoria, sedation).
Dosing and Administration
Gabapentin is rapidly absorbed and eliminated in both canine and feline patients.8 Frequent administration is needed to maintain minimum target plasma concentrations. 8 Pharmacokinetic data suggest that gabapentin should be dosed at 10 mg/kg every 8 hours in dogs, 8 and 8 mg/kg every six hours in cats.9
Administration of gabapentin on an as-needed basis or at intervals less frequent than indicated may result in insufficient plasma concentrations and lack of efficacy.
Gabapentin is typically considered a safe alternative to other medications, but its administration is not entirely without side effects. Sedation is a common adverse effect of gabapentin, particularly with administration at high doses.10,11 Ataxia is another common adverse effect of gabapentin,11 and administration in patients with pelvic-end weakness may exacerbate signs and decrease the ability to ambulate without assistance. Finally, gabapentin is removed from the body via the kidneys and should be used cautiously in patients with renal insufficiency, as increased adverse effects (e.g., sedation, hypotension) are possible.12-14
Why Prescribing Practices Matter
Gabapentin has recently become a popular street drug, with recreational users taking large doses to get high.15 The prevalence of abuse within the general population remains low, but it increases dramatically for people with a history of opioid abuse.15 Emergency departments (ED) are reporting a dramatic increase in the presence of gabapentin in the toxicology screens of individuals presenting to the ED for a drug overdose.16 Most concerning, patients who present to the ED following an overdose are significantly more likely to end up on a ventilator or have a fatal overdose if they have combined an illicit opioid with gabapentin.16,17
Conclusion
The use of gabapentin in veterinary medicine has increased dramatically in the last several years. Despite its popularity, there is a narrow indication of its use in veterinary patients. There is also growing evidence that gabapentin is being diverted for recreational drug use, sometimes with fatal consequences. As a result, the veterinary community should closely scrutinize where gabapentin fits into our “analgesic toolbox,” gabapentin should not be prescribed for conditions where it is unlikely to be effective. Additionally, veterinary practitioners should consider limiting the number of drugs dispensed and placing restrictions on refill authorizations.
x
References
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6(1):108-113.
- Kharasch ED, Clark JD, Kheterpal S. Perioperative gabapentinoids: deflating the bubble. Anesthesiology. 2020;133(2):251-254.
- Kuehn BM. Gabapentin increasingly implicated in overdose deaths. JAMA. 2022;327(24):2387. doi:10.1001/jama.2022.10100
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
- Reader R, Olaitan O, McCobb E. Evaluation of prescribing practices for gabapentin as an analgesic among veterinary professionals. Vet Anaesth Analg. 2021;48(5):775-781.
- Mathews K, Kronen PW, Lascelles D, et al. Guidelines for recognition, assessment and treatment of pain. J Small Anim Pract. 2014;55(6):E10-E68.
- Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002.
- Kukanich B, Cohen RL. Pharmacokinetics of oral gabapentin in greyhound dogs. Vet J. 2011;187(1):133-135. doi:10.1016/j.tvjl.2009.09.022
- Siao KT, Pypendop BH, Ilkiw JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res. 2010;71(7):817-821.
- Epstein M, Rodan I, Griffenhagen G, et al. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J Am Anim Hosp Assoc. 2015;51(2):67-84. doi:10.5326/JAAHA-MS-7331
- Guedes AGP, Meadows JM, Pypendop BH, Johnson EG, Zaffarano B. Assessment of the effects of gabapentin on activity levels and owner-perceived mobility impairment and quality of life in osteoarthritic geriatric cats. J Am Vet Med Assoc. 2018;253(5):579-585.
- Quimby JM, Lorbach SK, Saffire A, et al. Serum concentrations of gabapentin in cats with chronic kidney disease. J Feline Med Surg. 2022;1098612X221077017. doi:10.1177/1098612X221077017
- Blum RA, Comstock TJ, Sica DA, et al. Pharmacokinetics of gabapentin in subjects with various degrees of renal function. Clin Pharmacol Ther. 1994;56(2):154-159. doi:10.1038/clpt.1994.118
- Zand L, McKian KP, Qian Q. Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am J Med. 2010;123(4):367-373.
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
- Millar J, Sadasivan S, Weatherup N, Lutton S. Lyrica nights–recreational pregabalin abuse in an urban emergency department. Emerg Med J. 2013;30(10):874.
- Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.