-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Lisa Gorman, DVM, DACVIM
By Lisa Gorman, DVM, DACVIM
angell.org/internalmedicine
internalmedicine@angell.org
MSPCA-Angell West, Waltham
Chronic gastrointestinal (GI) signs, including weight loss, vomiting, diarrhea, and inappetence, are some of the most common reasons that cats present to the internal medicine service. Readily available diagnostics such as a chemistry panel, total T4, and fecal testing are important steps in ruling out many possible causes for these clinical signs. However, if these early diagnostics do not confirm a cause of the GI signs, diagnostic testing targeted to the gastrointestinal tract is warranted.
One early and minimally invasive diagnostic option that can be used in cats with chronic GI signs is a diet trial. This can use a low residue gastrointestinal diet, a novel protein diet, or a hydrolyzed diet, with the latter two options targeting animals that may have food sensitivity. Several studies have validated the use of low residue diets in cats with mild to moderate chronic GI signs, and unlike in dogs, dietary fat content does not appear to have an effect.1, 2 Novel protein diets have also been shown to have a positive effect in a large number of cats with idiopathic GI signs, with up to 50% having a positive response in one study.3 Unlike in dermatologic disease, response to diet trials in animals with GI disease usually occurs much more quickly, so diet trials of two weeks are adequate to determine effectiveness.
Measurement of serum cobalamin, folate, and potentially trypsin-like immunoreactivity (TLI) is warranted in many cases, although the frequency of abnormalities in cobalamin concentrations in cats with chronic enteropathies varies between studies.4, 5 Cobalamin deficiency strongly supports the presence of ileal disease or exocrine pancreatic insufficiency (EPI), while folate deficiency is indicative of duodenal disease. Although EPI is rare in cats, it is worth testing cats presenting primarily with weight loss for this disease, as many affected cats lack the classic clinical signs of diarrhea and polyphagia that are seen in dogs.6
When most systemic diseases have been ruled out, the next step for evaluation of cats with chronic GI signs is an abdominal ultrasound. In addition to looking for changes in the intestinal tract that support the presence of a chronic enteropathy, ultrasound can also find evidence of other disorders that could contribute to GI signs, such as gastrointestinal masses, pancreatitis, or hepatobiliary disease. Thickening of the intestinal wall in general and the muscularis layer in particular is associated with both lymphoma and inflammatory bowel disease (IBD), and mesenteric lymphadenopathy can also be seen in both conditions. One study suggested that the presence of both lymphadenopathy and muscularis thickening is more common in lymphoma and thus may increase suspicion for this disease, but does not rule out IBD.7 It is also worth noting that there are cats with IBD and lymphoma that have no ultrasonographic abnormalities, so a normal ultrasound cannot rule out either condition. If small intestinal thickening is seen, ultrasound is also useful in determining the most severely affected area, as this can guide what type of biopsy would be ideal for the patient. A cat with ultrasonographic abnormalities that are confined to the stomach and duodenum is an ideal candidate for endoscopy, while one with abnormalities that primarily affect the jejunum would likely have greater benefit from surgical biopsies to allow access to the affected area.
The ideal method of performing biopsies in cats is a contested issue, with some clinicians advocating for full thickness surgical biopsies in all cases, while others recommend endoscopy unless there is segmental disease that does not appear to affect the areas that can be reached with a scope (stomach, duodenum, and ileum). One study presented evidence that endoscopic biopsies may underdiagnose lymphoma compared with full thickness biopsies, but these results must be interpreted cautiously, as the endoscopic technique used in this study did not allow adequate duodenal evaluation in a large proportion of cats.8 The WSAVA International Gastrointestinal Standardization Group currently recommends that GI biopsies be obtained endoscopically in most cases, although they recommend performing gastric, duodenal, and ileal biopsies.9 The recommendation to perform both colonoscopy with ileal biopsies and gastroduodenoscopy in cases of suspected chronic small intestinal disease is based on evidence that lymphoma may be confined to the ileum in a significant number of cats. One study found that of cats with lymphoma that had endoscopic biopsies performed, 39% of cats had lymphoma confined to the duodenum, 44% had lymphoma confined to the ileum, and only 17% had lymphoma affecting both locations.10 While obtaining biopsies from both the upper and lower gastrointestinal tracts is ideal, sometimes gastroduodenoscopy alone is selected on the basis of practicality and cost, as preparation for a colonoscopy is more intensive and unpleasant for the patient.
Obtaining biopsies in cats with clinical signs of a chronic enteropathy is recommended, because several studies have shown that the vast majority of cats with these clinical signs and ultrasonographic evidence of intestinal thickening have underlying disease. One study found that 99% of cats that had chronic GI signs and thickened small intestinal walls on ultrasound examination had underlying pathology on full thickness biopsies. 49% of these cats had chronic enteritis (presumed IBD), and 46% had lymphoma. Importantly, 26% of cats evaluated in the study had initially presented for wellness exams in which the owner had mentioned chronic vomiting that they felt was normal for the cat, and all of these patients had disease, with 38% of them having lymphoma.11 This highlights the need to truly consider chronic vomiting a problem rather than a “normal” behavior for cats. A continuation of this study including a total of 300 cats showed similar results: 96% of cats had underlying pathology, with 50% of study cats having chronic enteritis and 41% having lymphoma.5
Once the underlying disease is diagnosed, appropriate treatment can be initiated. The treatment of choice for IBD in cats that did not respond to a diet trial is immunosuppressive steroid therapy with prednisolone. It is important to use prednisolone rather than prednisone in cats, as cats cannot consistently convert the prodrug prednisone to the active prednisolone. The recommended starting dose for IBD in cats is generally between 1 and 2 mg/kg/day, which is then tapered every 3-4 weeks depending on clinical response. In a cat who cannot receive systemic steroids due to other underlying disease, treatment with budesonide could be initiated at 0.5-1 mg per cat once daily, although budesonide use in cats is only anecdotal. As budesonide has extensive first pass hepatic metabolism, it has less systemic absorption and thus theoretically should have reduced systemic side effects. Treatment with chlorambucil can also be considered in patients who cannot receive steroids, or in patients who do not respond to steroids alone.
The most commonly used treatment protocol for small cell GI lymphoma is a combination of prednisolone (starting at 1-2 mg/kg/day and then tapered, ideally to every 48 hour dosing), and chlorambucil at 2 mg per cat every 48-72 hours. With this treatment protocol, the median survival time for cats with small cell GI lymphoma in one study was approximately 2 years.12 Alternatively, a chlorambucil dose of 20 mg/m2 given every 2 weeks has also been described.13 Although chlorambucil is generally well-tolerated, periodic monitoring of CBCs is recommended to ensure there are no negative side effects.
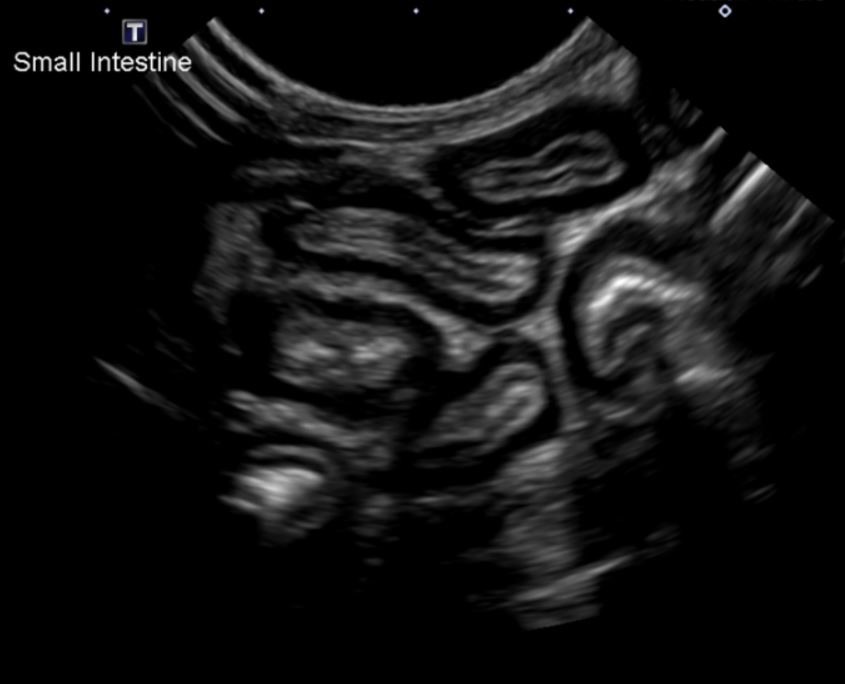
Figure 1: Diffusely thickened small intestinal loops on ultrasound examination. Note the markedly thickened muscularis layer in all pictured intestinal segments.
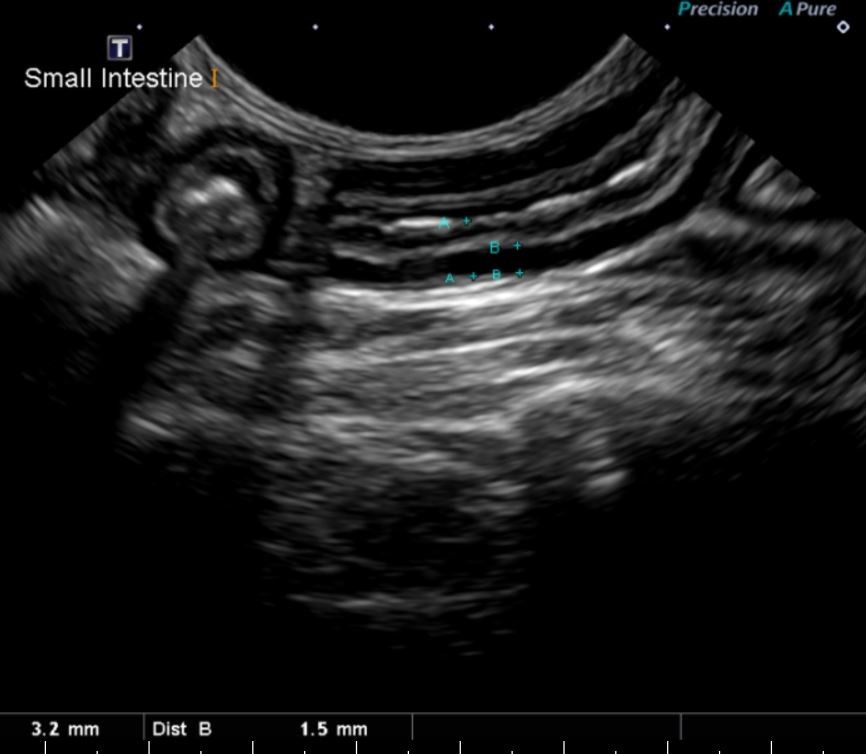
Figure 2: Image of a small intestinal segment in the same cat with measurements. Note the overall thickening of the small intestine at 3.2 mm (normal is < 2.5 mm) and the thickness of the muscularis layer in particular at 1.5 mm.
Chronic enteropathies are a common clinical problem in cats, and in most cases are due to either inflammatory bowel disease or small cell GI lymphoma. Both of these conditions can be effectively treated, with average survival times of years even for small cell lymphoma. This highlights the importance of pursuing diagnostics in these cats so that their disease can be managed appropriately, even in cases where the owner considers vomiting a “normal” part of the cat’s life. Management of the underlying disease results in a good response in most cases with improved quality of life.
References:
- Perea et al. Evaluation of two dry commercial therapeutic diets for the management of feline chronic gastroenteropathy. Frontiers in Veterinary Science. May 2017; 4 (69).
- Laflamme et al. Effect of diets differing in fat content on chronic diarrhea in cats. J Vet Intern Med 2011; 25: 230-235.
- Grant Guilford et al. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J Vet Intern Med 2001; 15: 7-13.
- Maunder et al. Serum cobalamin concentrations in cats with gastrointestinal signs: correlation with histopathological findings and duration of clinical signs. J Feline Med Surg 2012; 14 (10): 686-693.
- Norsworthy et al. Prevalence and underlying causes of histologic abnormalities in cats suspected to have chronic small bowel disease: 300 cases (2008-2013). J Am Vet Med Assoc 2015; 247: 629-635.
- Xenoulis et al. Feline exocrine pancreatic insufficiency: A retrospective study of 150 cases. J Vet Intern Med 2016; 30: 1790-1797.
- Zwingenberger et al. Ultrasonographic evaluation of the muscularis propria in cats with diffuse small intestinal lymphoma or inflammatory bowel disease. J Vet Intern Med 2010; 24: 289-292.
- Evans et al. Comparison of endoscopic and full thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc 2006; 229: 1447-1450.
- Washabau et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010; 24: 10-26.
- Scott et al, JVIM 2011; Utility of endoscopic biopsies of the duodenum and ileum for diagnosis of inflammatory bowel disease and small cell lymphoma in cats. J Vet Intern Med 2011; 25: 1253-1257.
- Norsworthy et al. Diagnosis of chronic small bowel disease in cats: 100 cases. J Am Vet Med Assoc 2013; 243: 1455-1461.
- Kiselow et al. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995-2005). J Am Vet Med Assoc 2008; 232: 405-410.
- Vail, David. Feline lymphoma and leukemia. Withrow and MacEwen’s Small Animal Clinical Oncology, Fifth Edition. Eds Stephen Withrow, David Vail, and Rodney Page. St. Louis: Saunders 2013. 638-653.