-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 Maureen Carroll DVM, DACVIM
Maureen Carroll DVM, DACVIM
angell.org/internalmedicine
internalmedicine@angell.org
617-541-5186
In Humans and animals, hypovitaminosis D is presently a topic receiving close review in the etiology of multisystemic disease including gastrointestinal disease, cancer, heart and infectious disease, as well as diseases that involve chronic inflammation.
Vitamin D consists of 2 bioequivalent forms. Vitamin D2 also known as ergocalciferol, is obtained from dietary vegetable sources and oral supplements. Vitamin D3 also known as cholecalciferol, is obtained primarily from skin exposure to ultraviolet B (UVB) radiation in sunlight, or ingestion of food sources such as fish, egg yolk, butter, soy and liver, as well as oral supplements. Most studies show that vitamin D3 is more effective than vitamin D2 at raising blood levels of vitamin D. Humans and some other mammals (rats/ sheep) can produce vitamin D3 in the skin, however dogs and cats are unable, and must rely exclusively on diet for vitamin D, which is why the ingredients used in pet food are a rich source of vitamin D3.
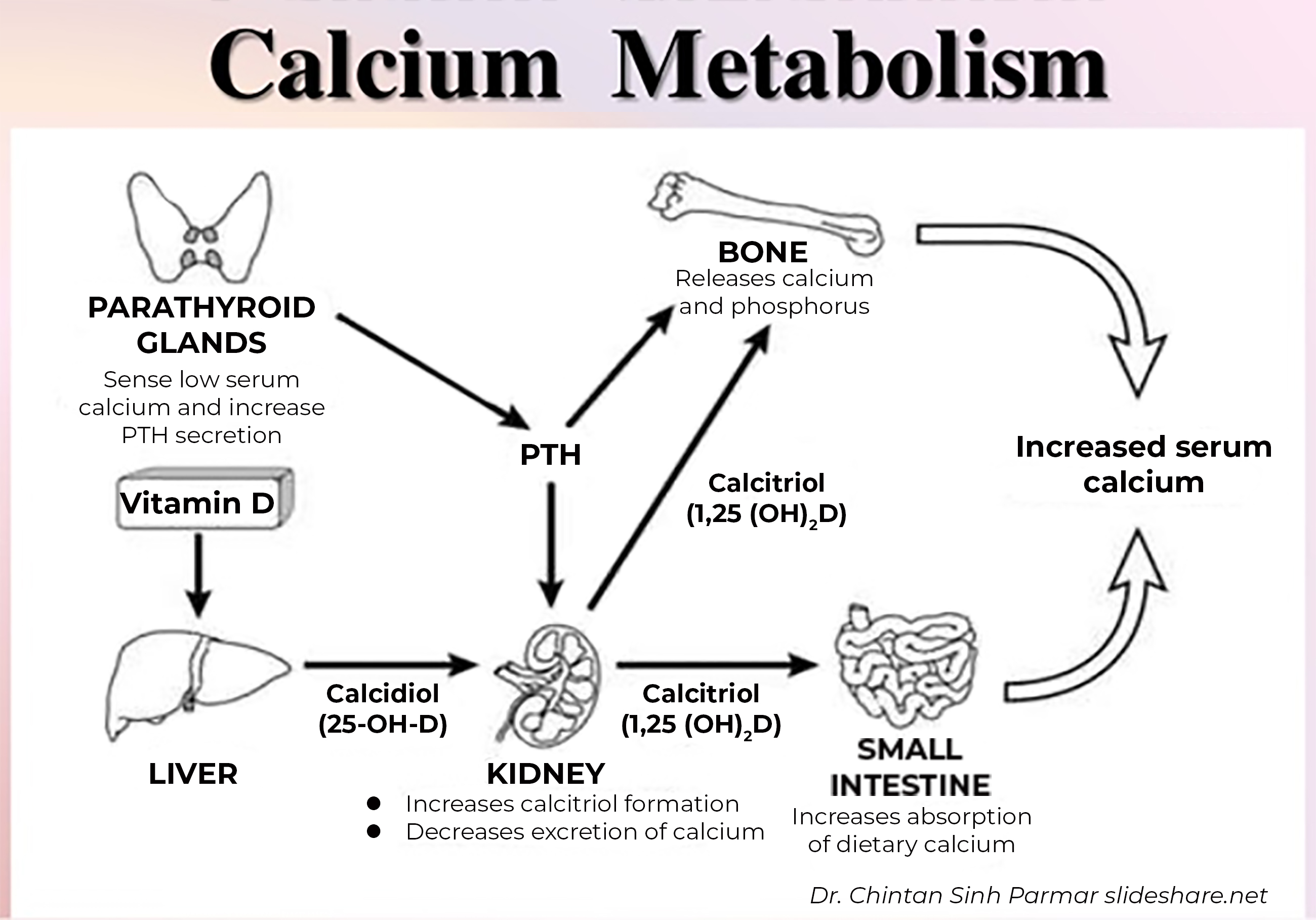
Vitamin D from the diet or skin synthesis is biologically inactive; enzymatic conversion (hydroxylation) in the liver and kidney is required for activation. Once vitamin D enters the body, it is absorbed in the intestine at which point it becomes stored in fat, or is transported to the liver. In the liver, vitamin D is hydroxylated to 25-hydroxyvitamin D [25(OH)D], composed of both forms of vitamin D: 25(OH)D2 and 25(OH)D3. 25(OH)D (also called calcidiol) is subsequently converted to 1,25-dihydroxyvitamin D [1,25(OH)2D] in the kidney and select other tissues by the action of the 1α-hydroxylase enzyme. The resultant 1,25 di- hydroxy vitamin D is the most physiologically active form, also known as calcitriol. The main targets for vitamin D are the intestine, bone, kidney, and parathyroid glands.
The main function of vitamin D is to maintain calcium and phosphorus homeostasis by increasing intestinal absorption of calcium and phosphorus, mobilizing calcium and phosphorus from bone and stimulating Ca reabsorption and phosphorus excretion in the kidney. In addition, In the kidney, 1,25(OH)2D inhibits renal 1α-hydroxylase and stimulates 24-hydroxylase (which degrades vitamin D), thereby reducing production and increasing catabolism of 1,25(OH)2D.
The importance of vitamin D in the functioning of many body systems is the topic of current research being conducted today. In the gastrointestinal (GI) tract, we can observe low vitamin D levels as a results of decreased intake, or loss as in the case of inflammatory conditions of the bowel. However when dogs with chronic enteropathies were compared to dogs with non-GI illnesses (independent of vitamin D intake), their vitamin D levels were still lower suggesting other mechanisms involved with hypovitaminosis D. These low levels of D were associated with a decrease in both total and ionized calcium, low albumin, as well as a poorer prognosis. Therefore although hypovitaminosis D in chronic enteropathies has traditionally been considered to be a result of intestinal disease, there is growing evidence that hypovitaminosis D may contribute to the initiation of intestinal inflammation rather than just being a secondary consequence of the intestinal pathology.
In a recent study looking at serum vitamin D levels and markers of inflammation in dogs with chronic enteropathies, vitamin D levels were inversely related to the inflammatory marker levels. It has been postulated vitamin D deficiency contributes to the development of IBD as we know it can affect tight junctions and epithelial permeability, thereby affecting antigen exposure and the mucosal immune system.
In addition, a recent study, which explored the relationship between vitamin D status and inflammation in dogs with chronic enteropathies, a negative relationship was discovered between serum 25(OH)D concentrations and neutrophil and monocyte concentrations, serum IL-2 and IL-8 concentrations, duodenal inflammatory scores.
Finally, supporting evidence for a link between hypovitaminosis D and chronic enteropathies comes from rodent models that have demonstrated vitamin D receptor-deficient mice are more susceptible to experimental forms of inflammatory bowel disease. Additionally, feeding mice a diet that is vitamin D deplete predisposes them to the development of colitis. Research is ongoing regarding the role of hypovitaminosis D and its contribution to the intestinal inflammation.
Vitamin D has also been shown to be related to cardiovascular disease. A recently published study investigated the relationship between vitamin D status, represented by serum 25(OH)D measurements, and congestive heart failure (CHF) or cardiovascular events in 82 client-owned dogs. Mean 25(OH)D concentrations were lower in dogs with CHF compared to unaffected dogs, regardless of dietary vitamin D intake and there was an association between 25(OH)D concentrations and the time to clinical manifestation of CHF or sudden death. In addition, mean calculated vitamin D intake per kg of metabolic body weight in dogs with congestive heart failure was not significantly different from that of unaffected dogs. Another study demonstrated that median 25(OH)D concentrations were significantly lower in dogs with valvular heart disease stage B2 and C/D disease than dogs with stage B1 disease.
Vitamin D deficiency has been associated with human cardiovascular diseases, including myocardial infarction and sudden cardiac death. The pathogenesis of hypovitaminosis D might be related to D’s effect on promoting cardiac contractility / and having an anti- hypertrophic effect on myocardial cells.
Neoplasia is probably one of the more studied areas involving vitamin D metabolism and its effect on cancer development and metabolism. 25(OH)D measurements in a group of 87 Labrador Retrievers showed that mean serum 25(OH)D concentrations were lower in dogs with cutaneous mast cell tumors, compared to unaffected dogs, independent of dietary vitamin D intake. The low vitamin D status in dogs with a tumor may be of significance since the vitamin D receptor is expressed on the majority of mast cell tumors. In humans, low 25 (OH)D concentrations have been associated with an increased incidence of cancer. As it is estimated that 25% of cancers are a result of chronic inflammation, perhaps there is a relationship between Vitamin D as it relates to chronic inflammatory conditions.
Chronic inflammatory conditions of the kidneys, heart, GI tract, as a result of chronic infection have been linked to low vitamin D levels. Low vitamin D status has been negatively associated with C-reactive protein in dogs with hemoabdomen; other studies have uncovered abnormalities in cellular function as well as cytokine levels as a result of low D levels.
Infections in humans such as mycobacteria (and in cats), influenza, tuberculosis and HIV; and Spirocerca lupi infections in dogs, are the focus of vitamin D research. Vitamin D has been used to treat chronic mycobacteria infection as a link has been discovered between low levels of D and the immune response to this infection. In cats with mycobacterial infections, lower levels of 25 (OH) D were discovered, and in dogs with Spirocerca lupi lower vitamin D levels were detected as compared to healthy dogs. Another study investigating calcium homeostasis in dogs with endotoxemia demonstrated that ionized hypocalcemia occurred in conjunction with hypovitaminosis D after intravenous endotoxin administration. Additionally, a canine sepsis model of induced Staphylococcus pneumonia demonstrated that serum D2 measurements were predictive of shortened survival. Unfortunately, investigations in critically ill, client-owned veterinary patients or those with naturally occurring sepsis are lacking.
Vitamin D measurement/ Vitamin D treatment:
Measurement of the monohydroxylated form of vitamin D3 is the widely accepted as the best indicator of vitamin D status due to its long half‑life and higher concentrations in circulation. Currently a radioimmunoassay (RIA) is used to measure vitamin D levels in cats and dogs. This assay dose not differentiate between D2 and D3 (which may be important in the research setting), and in addition there is nonspecific binding to other vitamin D metabolites that have similar structures, resulting in overestimation of the vitamin D concentration. The current gold standard to measure D in human medicine is the liquid chromatograph‑tandem mass spectrometry (LC-MS/MS) which eliminates the aforementioned challenges in the RIA. Presently I am investigating running paired samples for vitamin D levels using the standard RIA used in veterinary medicine, and comparing to LC/ MS/ MS.
Much remains to be learned in terms of the beneficial effects of vitamin D supplementation on outcome. Many researchers caution that while vitamin D deficiency and increased morbidity and mortality appear closely associated and even though vitamin D has many beneficial effects within the body, there are too many knowledge gaps and a lack of prospective studies to determine when and how to supplement vitamin D. In veterinary medicine, one question is whether to dose vitamin D3 (which requires activation/ hydroxylation in the liver and kidney) or calcitriol, which does not require activation. Further evidence for vitamin D supplementation is required before recommendations can be made in the various disease states discussed above.
References
- H Titmarsh, A G Gow, S Kilpatrick et al J Vet Intern Med. 2015 Nov-Dec; 29(6):1473-8
- K A Selting, C R Sharp, R Ringold et al J Vet Comp Oncol. 2016 September; 14(3):295-305
- M S Kraus, K M Rassnick, J J Wakshlag et al; J Vet Intern Med. 2014 Jan-Feb; 28(1):109-15.
- Kraus MS, Rassnick KM, Wakshlag JJ, Gelzer ARM, Wasman AS, Struble AM, et al J Vet intern Med. 2014,; 28 (1): 109-15
- Joseph J Wakshlag, Kenneth M Rassnick, Erin K Malone et al; Br J Nutr. 2011 October; 106 Suppl 1(0):S60-3.
- Mellanby R, Mellor P, Roulois A, Baines E, Mee A, Berry J, et al. J Small Anim Pract. 2005;46(7):345–51.
- Gow AG, Else R, Evans H, Berry JL, Herrtage ME, Mellanby RJ. J Small Anim Pract. 2011;52(8):411–8.
- Lalor S, Schwartz AM, Titmarsh H, Reed N, Tasker S, Boland L, et al. J Vet Intern Med. 2014.
- Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. Mol Endocrinol. 2003;17(12):2386–92.
- Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Endocrinology. 2010;151(6):2423–32.
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Circulation. 2008;117(4):503–11.
- Kendrick J, Targher G, Smits G, Chonchol M. Atherosclerosis. 2009;205(1):255–60.
- Weber KT, Weglicki WB, Simpson RU. Cardiovasc Res. 2009;81(3):500–8.
- Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. JMCC. 2006;41(2):350–9.
- Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, et al. PNAS. 2-12; 109 (38): 1529-54
- Osuga T, Nakamura K, Morita T, Lim SY, Nisa K, Yokoyama N, et al. J Vet Intern Med. 2015.
- Cassidy JP, Martineau AR. J Comp Pathol. 2014.
- A G Gow, R Else, H Evans et al; J Small Anim Pract. 2011 August; 52(8):411-8.
- Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, et al. JCI 2007;117(7):1988–94.
- Rosa CT, Schoeman JP, Berry JL, Mellanby RJ, Dvir E. J Vet Intern Med 2013;27(5):1159–64.
- Wakshlag JJ, Rassnick KM, Malone EK, et al. Brit J Nutr. 2011;106 Suppl 1:S60–3.
- Russell DS, Rassnick KM, Erb HN, Vaughan MM, McDonough SP. J Comp Pathol. 2010;143(2–3):223–6.
- Gerber B, Hassig M, Reusch CE. Am J Vet Res. 2003;64(9):1161–6.
- Selting KA, Sharp CR, Ringold R, Thamm DH, Backus R. Vet Comp Oncol. 2014:n/a-n/a.
- Titmarsh HF, Gow AG, Kilpatrick S, Cartwright JA, Milne EM, Philbey AW, et al. PLOS One. 2015;10(9):e0137377.