-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 Pamela Mouser, DVM, MS, DACVP
Pamela Mouser, DVM, MS, DACVP
Anatomic Pathologist
angell.org/lab
Cutaneous and subcutaneous masses are among the most common surgical samples submitted for histopathology. As with any tumor type, the clinical questions to be answered for skin masses include: What is it? How will it behave? Is it completely excised? At the microscope, the pathologist must first determine if the mass is truly neoplastic—which can be surprisingly challenging on occasion!—and then further characterize the process based on histogenesis, or tissue differentiation. Most pathologists stratify neoplasms into three groups using morphology: epithelial, mesenchymal/spindloid, or round cell origin. Poorly pigmented melanocytic tumors may overlap any or all of these morphologies, and therefore may represent their own (fourth) group. This article will focus specifically on round cell tumors of the skin, including percentages of each tumor type relative to the total cutaneous/subcutaneous round cell tumors submitted to Angell in the past 4.5 years.
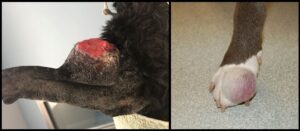
Mast cell tumor
More than 80% of canine round cell tumors in the skin and subcutis evaluated histologically in this recent Angell population are mast cell tumors. In cats, the proportion of mast cell tumors approaches 80%. Canine mast cell tumors can present with a range of gross manifestations, including a discrete raised dermal nodule, a plaque-like thickening which is minimally raised, or a dermal to subcutaneous mass with or without discrete margins (Figure 1). Alopecia (from displacement of adnexal structures by neoplastic cells) and ulceration are common, as is a history of fluctuant tumor size. Histologically, the majority of canine mast cell tumors do not present a diagnostic challenge as the neoplastic mast cells are often well-differentiated or have discernible granules. Eosinophils, which frequently accompany neoplastic canine mast cells, are a helpful reminder to assess for the presence of granules in poorly-differentiated tumors. Cutaneous mast cell tumors in dogs are graded using one or both published grading schemes (three-tier system established by Patnaik et al in 1984 or two-tier system by Kiupel et al in 2011), in an attempt to predict biological behavior for a given mass.5,6
Figure 1 depicts two cutaneous mast cell tumors. In both examples, the tumors appear nodular and protrusive. The mass on the left has ulceration of the overlying skin surface, while the mass on the right appears alopecic due to separation and possibly disruption of hair follicles by infiltrating mast cells.
Feline cutaneous mast cell tumors often occur as single, discrete, raised skin nodules. In contrast to dogs, feline mast cell granules are not as visually pronounced with routine stains. In addition, feline mast cell tumors may have few or no accompanying eosinophils. A recent study suggests application of a two-tier grading system for feline mast cell tumors.8 A low-grade designation using this system essentially corresponds to benign biological behavior, as is commonly predicted for well-differentiated feline cutaneous mast cell tumors. Feline mast cell tumors classified as high-grade in this study had a median survival time of 349 days.
Plasma cell tumor
Cutaneous plasma cell tumor (plasmacytoma) is the next most frequent diagnosis in dogs during this timeline (about 8% of canine cutaneous round cell tumors). Grossly, most cutaneous plasma cell tumors resemble cutaneous histiocytomas (discussed below), and are characterized by a discrete raised alopecic dermal nodule (Figure 2). Also similar to histiocytomas, plasma cell tumors favor the skin of the head and distal extremities. Microscopically, these tumors commonly have binucleate, multinucleate, and karyomegalic cells distributed among the background mononuclear population. Plasmacytoid features (eccentric clock-faced nuclei with paranuclear pallor) may be seen in some neoplastic cells, particularly near the margins of the mass. In tumors where plasmacytoid differentiation is not appreciated and nuclear pleomorphism is pronounced, morphology may suggest a more aggressive lesion such as histiocytic sarcoma. Cutaneous plasma cell tumors typically have benign biological behavior in dogs and are rarely associated with multiple myeloma. Dogs presenting with multiple cutaneous plasma cell tumors, termed cutaneous plasmacytosis, may experience shorter survival times according to one retrospective study.1 Cutaneous plasma cell tumors are rare in cats.
Canine cutaneous histiocytoma
Cutaneous histiocytomas represent about 4% of cutaneous round cell tumors from dogs in recent Angell submissions. This percentage likely underestimates the relative proportion of this tumor type, as histiocytomas may be more commonly addressed by primary care veterinarians and therefore not removed as often by Angell’s surgery service. This tumor is generally considered a lesion of young dogs, although affected animals in the Angell group range from 1-12 years old with a median of 6 and mean of 6.2 years old. Cutaneous histiocytomas, sometimes referred to as “button” tumors due to their discrete raised nodular gross appearance, have benign biological behavior (Figure 2). Spontaneous regression may occur, although the associated ulceration and inflammation may require surgical intervention. Regressing histiocytomas are sometimes indistinguishable from mononuclear inflammation, and may be diagnosed as such by the pathologist.
Figure 2 compares cutaneous plasma cell tumor (plasmacytoma, left) and cutaneous histiocytoma (right). Both lesions are discrete, raised, alopecic masses. Erythema around the plasma cell tumor on the left may be related to self-trauma or clinical manipulation of the lesion. The amputated distal tail on the right has been formalin-fixed with serial slices through the mass for fixation.
Lymphoma
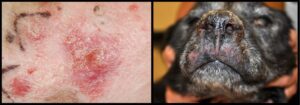
About 4% of canine cutaneous round cell tumors in this population are classified as lymphoma, with the majority of these tumors demonstrating epitheliotropism (epithelial affinity by neoplastic lymphoid cells). Cutaneous epitheliotropic lymphoma is typically of T-cell origin, and is also called mycosis fungoides. Grossly, lesions may be solitary, multifocal, or widespread and have a variable clinical appearance which may include scaling, erythema, thickened skin, erosion/ulceration, or nodules (Figure 3). Mucocutaneous junctions and mucous membranes may be affected. Histologically, neoplastic lymphoid cells in epitheliotropic lymphoma are small to medium size and form nests which may coalesce into broad plaques within the epidermis and adnexal structures. In a recent retrospective study of dogs with epitheliotropic lymphoma, the median survival time for dogs with cutaneous lesions was 130 days.3 Dogs presenting with a solitary skin lesion had a better prognosis (median survival time of 231 days), as did dogs with mucocutaneous/mucosal lesions (median survival time of 491 days). Chemotherapy also significantly increased the median survival time in the retrospective study.
Figure 3 shows two manifestations of cutaneous lymphoma in dogs. The left panel is a close-up view of affected abdominal skin showing erythema, scaling, and raised plaques to nodules. The black V-shaped marks were made by the clinician to guide the biopsy procedure. The dog on the right has thickening, crusting, and cracking of the nasal planum. Skin of the upper lip is also thickened with multifocal to coalescing ulceration. Note how gross lesions of cutaneous lymphoma may overlap with those of non-neoplastic dermatopathies.
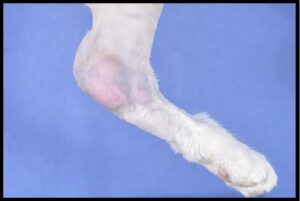
Lymphoma comprised about 9% of feline cutaneous round cell tumors in the Angell population. In contrast to dogs, cutaneous lymphoma in cats is more often nonepitheliotropic. Cutaneous/subcutaneous lymphoma has been reported at injection sites in cats,7 and a seemingly unique variant of cutaneous lymphoma develops over the tarsus.2 Cats with tarsal lymphoma in the published retrospective study frequently had or developed metastatic disease. The median survival time for cats with tarsal lymphoma was 190 days. Interestingly, more than half of the Angell feline cutaneous lymphoma submissions were compatible with tarsal lymphoma (Figure 4).
Figure 4: This left hind limb from a cat has a multinodular subcutaneous mass surrounding the tarsal joint. Histologically, the mass is composed of neoplastic large lymphoid cells, which also distend sinuses in the left popliteal lymph node.
Histiocytic sarcoma
Between 2-3% of canine cutaneous/subcutaneous round cell tumors from the past 4.5 years were diagnosed as histiocytic sarcoma. This malignant tumor of histiocytes typically presents a single expansile subcutaneous mass, often on the extremities, with a clinical appearance similar to a subcutaneous mast cell tumor or soft tissue sarcoma. In fact, neoplastic cells may range from round to ovoid to spindloid histologically, and may ultimately require immunohistochemistry to confirm their leukocytic origin. Histiocytic sarcoma should also be distinguished from cutaneous/reactive histiocytosis and granulomatous inflammation. In one retrospective study evaluating prognosis of histiocytic sarcoma, the authors noted that many tumors originally suspected to be of histiocytic origin were excluded following immunohistochemistry.4 In the same study, localized histiocytic sarcoma (which is the common presentation for cutaneous/subcutaneous histiocytic sarcoma) had a median survival time around 1 year. Histiocytic sarcoma is less common in cats, with few suspect cases (unconfirmed) in the Angell population.
Final take home points
Cutaneous round cell tumors range from potentially self-resolving benign lesions (canine cutaneous histiocytoma) to aggressive malignancies (cutaneous lymphoma). While distinction between these entities is often relatively clear-cut, cell of origin may not be immediately obvious on histopathology. Fine needle aspirates or impression smears of the biopsy may prove valuable to the pathologist, as cytologic preparations may retain cellular detail in better quality. Do not hesitate to pursue recommended ancillary diagnostic tests (special stains, immunohistochemistry, or PCR for antigen receptor rearrangement [PARR]) to more completely classify a cutaneous round cell tumor, as the behavior and prognosis differs widely within this group.
References
- Boostrom BO, Moore AS, DeRegis CJ, et al. Canine cutaneous plasmacytosis: 21 cases (2005-2015). J Vet Intern Med 2017;31:1074-1080.
- Burr HD, Keating JH, Clifford CA, Burgess KE. Cutaneous lymphoma of the tarsus in cats: 23 cases (2000-2012). J Am Vet Med Assoc 2014;244:1429-1434.
- Chan CM, Frimberger AE, Moore AS. Clinical outcome and prognosis of dogs with histopathological features consistent with epitheliotropic lymphoma: a retrospective study of 148 cases (2003-2015). Vet Dermatol 2018;29:154-e59.
- Dervisis NG, Kiupel M, Qin Q, Cesario L. Clinical prognostic factors in canine histiocytic sarcoma. Vet Comp Oncol 2017;15:1171-1180.
- Kiupel M, Webster JD, Bailey KL, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol 2011;48:147-155.
- Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol 1984;21:469-474.
- Robbabianca P, Avallone G, Rodriguez A, et al. Cutaneous lymphoma at injection sites: pathological, immunophenotypical, and molecular characterization in 17 cats. Vet Pathol 2016;53:823-832.
- Sabattini S, Bettini G. Grading cutaneous mast cell tumors. Vet Pathol 2019;56:43-49.