-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Klaus Loft, DVM
By Klaus Loft, DVM
angell.org/dermatology
617-524-5733
Even since the large and exhaustive review published by Dr. Muller et al in early 2012, “Treatment of demodicosis in dogs: 2011 clinical practice guidelines”[1] there have been several new treatments and diagnostics introduced into our clinical reality necessitating a short update on the diagnosis and treatment of canine demodicosis.
This update will start with a brief history and background on canine demodicosis followed by a brief review of the latest published insight into the demodex mites, and lastly, a brief review of current treatments.
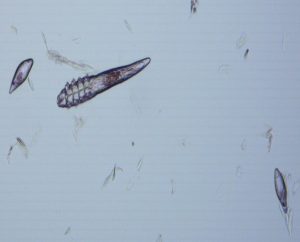 Historically described in 1842 as an obligate parasite of the pilosebaceous glands of humans; we consider the demodex canis, d. injai and d. cornei to be part of the normal canine skin microbiome. Mites are transferred via maternal contact very early in life to the offspring, as offspring born via cesarean and kept isolated will have no demodex mites and the mite is not considered contagious. The different canine demodex mites are closely related with no known cross species zoonotic risk but are, according to newer publications, genetically different. The full phylogenetic classification continues to evolve.
Historically described in 1842 as an obligate parasite of the pilosebaceous glands of humans; we consider the demodex canis, d. injai and d. cornei to be part of the normal canine skin microbiome. Mites are transferred via maternal contact very early in life to the offspring, as offspring born via cesarean and kept isolated will have no demodex mites and the mite is not considered contagious. The different canine demodex mites are closely related with no known cross species zoonotic risk but are, according to newer publications, genetically different. The full phylogenetic classification continues to evolve.
The proliferation of the mites in patients displaying dermatologic symptoms is thought to be a genetic and/or immunological issue, with lack of appropriate inflammatory response and disease control. The mites cannot survive in-vivo which complicates research and full understanding of the pathobiology of the mite. Each mammalian species have their own species of demodex mites making it difficult to extrapolate findings.
Clinical symptoms arise when the patients experience an excessive proliferation of the mites in the hair follicles and sebaceous glands resulting in an inflammatory skin disease. The inflammation is often complicated by secondary bacterial and/or yeast infections. In dogs, generalized demodicosis is differentiated clinically with the localized form which is defined by having less than 5 sites affected. Demodicosis can be further subdivided based on age of onset: juvenile-onset (onset of symptoms under age 18 months) or adult-onset. The localized form resolves spontaneously in most cases and treatment does not appear to accelerate the healing process. Based on the published “clinical practice guidelines[i]” treatment with a miticidal agent is not recommended in localized demodicosis as it is possible, that in a small number of cases, the localized demodicosis will progress into generalized demodicosis and this development could be “hidden” if all localized patients are treated. Generalized canine demodicosis is considered hereditary and can become a very severe skin disease, often complicated by secondary bacterial and/or yeast infections. In very severe cases this can lead to sepsis and patient death. This generalized form of demodicosis will need to be treated intensively and appropriately and in several countries, the breeding of animals suffering from hereditary generalized demodicosis is not recommended and actually may be prohibited by national breeding associations.
Diagnosis of the mites is classically done via multiple deep skin scrapings from affected areas of skin (alopecic, seborrhea sicca/oleosa pustules and furuncles). A dulled scalpel or spatula with mineral oil can be used to collect skin scrapings from patients. The sample site should be squeezed to raise the mites closer to the skin surface and the scraping made until capillary bleeding is noted on the scraped skin. The collected material is then microscopically examined at low magnification 4x to 10x with the condenser lowered and the light source turned down. If done appropriately, the diagnosis is straight forward. Areas close to lip margins, eyes, webbed skin on paws or areas with deep complicated pyoderma can be difficult to scrape. Plucking hair and a tape squeeze technique can be helpful in these harder-to-sample locations and will, if done well, yield a good diagnostic return.
Treatment of canine demodicosis is designed to resolve inflammation and thereby reduce any secondary skin infections and/or fur loss and ideally to remove the mites from the skin. The majority of localized cases will spontaneously resolve with no antiparasitic treatments needed. Generalized demodicosis is not necessarily a marker of general immunosuppression (Cushing’s syndrome, hypo-thyrodism, neoplastic malignancy or immune suppressive treatments). In patients with demodicosis and a comorbidity of immune altering conditions the resolution of the parasites will often be faster with fewer setbacks if the immune altering condition can be controlled. The gold standard of treatment is to reach two negative skin scrapings from previously affected sites within a 30 day interval. Treatments should be tailored to fit the patient’s signalment and to avoid using protocols interfering with medications needed for any concurrent health issues (seizures, MDR-1 defect, or food adverse reaction). Many commonly used demodex treatments are not registered specifically for canine demodicosis by the FDA or the EPA (e.g. Ivomec, Milbemycin). Several of the newer products have started to receive labeling for demodicosis (at least in Europe) or have been shown to have good antiparasitic effect in recent peer-reviewed publications.
Amitraz (Mitaban®) dip: Mitaban dip immersion bath is a FDA approved drug for this disease, but toy and tea cup breeds should not receive full treatment concentration. Dips are usually applied either weekly or every two weeks. Whole body clipping is often required for long-haired dogs throughout treatment so that the dip solution can reach the mites down in the hair follicle. Side effects of Mitaban include sedation, decreased body temperature, loss of appetite, vomiting, diarrhea and the odor of the dips is not pleasant. Treatment with an antidote, Yohimbine, can be used to decrease the severity of some Mitaban dip side effects. Eye lubrication may be needed if significant conjunctivitis is a problem of treatment. Spot-on Amitraz products (e.g., Certifect®) used primarily for flea and tick prevention also have some reported effect on demodicosis, and an earlier similar product (Promeris) was discontinued after an association with outbreaks of pemphigus was shown to occur in a small number of patients treated.
Lyme Sulfur dips can be used for demodicosis and are particularly effective in the earlier phases of treatment of very severe cases of generalized demodicosis with severe furuncolosis forming deep pyoderma. Its low cost and minimal toxicity makes it a safe but foul-smelling option in very young animals and animals at risk for toxicity or side effects as well as in cases with multi ectoparasitic disease.
Ivermectin (Ivomec®, Eqvalan®) is available as an injectable liquid or oral paste as a deworming agent for production animals. It can be given orally daily as a liquid to dogs to treat demodicosis. Subcutaneous injections will often cause injection site discomfort and can result in sterile abscess formation after long term use. Some dogs, especially herding breed dogs and animals with drugs affecting the blood brain barrier p-glycoprotein function, can have central nervous system sensitivity to the higher doses of ivermectin (MDR-1 or ABCB-1 gene defect) needed for demodex treatments. Signs of ivermectin sensitivity include sedation, drooling, loss of balance, vomiting, seizures, and, rarely, blindness. A genetic test is available to help determine if an individual dog may be sensitive to ivermectin. Dogs are first started on a low dose of ivermectin and then gradually given a higher dose while monitoring for side effects. Antifungal azoles such as fluconazole, itraconazole, and ketoconazole can affect the absorption, clearance and metabolism of ivomec.
Doramectin® and Moxidectin (Cydectin®, Advantage Multi®) is available as a liquid deworming (pour-on and injectable) agent for sheep and cows and as part of canine flea and heartworm preventive spot-on products. It can be given daily as an oral liquid to dogs to treat demodicosis and is also available as part of a spot-on product (Advantage Multi®) for weekly application, but is only registered for demodicosis outside the US. This product can be used in ivomec sensitive breeds as it is not a p-glycoprotein substrate, but other forms of neurotoxic like side effects have anecdotally been reported with the use of the injectable form.
Selamectin (Revolution®) topically is less effective although a few studies looking at oral use (evaporated on bread to avoid carrier vehicles) have shown some efficacy, but it is likely not the most practical option in clinical settings.
Milbemycin oxime (Trifexis®, Sentinel®, and Interceptor®) is available as a heartworm preventive pill for dogs. It can be given daily to dogs to treat demodicosis. Some breeds can have central nervous system sensitivity to high doses of milbemycin, but this is less common than a sensitivity to ivermectin. Signs of milbemycin sensitivity include sedation, drooling, loss of balance, vomiting, seizures, and rarely blindness. Cost will often become an issue.
Fluralaner; (Bravecto™, Nexgard®, and Simpirica®) is a new class of antiparasitic drugs targeting fleas and ticks, but lately has been shown to have a promising effect on some forms of sarcoptic and demodectic mange in dogs. Depending on the product, the treatment will have to be repeated monthly or every 90 days until the two negative skin scrapings have been accomplished.
With more findings in PCR and DNA sequencing of mites as well new treatments for canine demodicosis the possibilities are promising from the perspective of clinicians. With more options of treatments, it will likely allow us to tailor the demodicosis treatments to high risk patients, for example with known adverse reactions to antiparasitic classes of drugs or with complex health issues (seizure disorders, liver disease, etc.) We see this as a good opportunity to work with clients and hopefully also breeders so we can find ways to reduce the overall incidence of this disease in our pets.
- [1] Free full article download Muller, R., Bensignor, E., Ferrer, L., Holm, B., Lemarie, S., Paradis, M. & Shipstone, M.: “Treatment of demodicosis in dogs: 2011 clinical practice Guidelines”. Veterinary dermatology 2015. 1 1111/j.1365-3164.2011.01026.x
- Chapter 4.1.3 thru 4.1.3.3.2 in Mecklenburg, L. Linek, M. & Tobin, D.: “Hair Loss Disorders In Domestic Animals” p.204-09. Wiley-Blackwell, Iowa 2009.
- Chapter 6 in Muller & Kirk’s Small Animal Dermatology 7th p.304-13
- Yas-natan E., Shamir m., Kleinbart S., Aroch L.: “Doramectin toxicity in a collie” Vet Rec (2003)153,718-72
- Chapter 6 in Muller & Kirk’s Small Animal Dermatology 7th p.304-13
- Fourie J. et al; “Efficacy of orally administered Fluralaner (BravectoTM) or topically applied imidacloprid/moxidectin (Advocate®) against generalized demodicosis in dogs”. Journal of Parasites & Vectors (2015) 8:187.
- Plumbs veterinary drug handbook 8th