-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Nathalie Suciu, VMD
By Nathalie Suciu, VMD![]()
angell.org/emergency
emergency@angell.org
617-522-7282
April 2023
x
x
x
Immune-mediated polyarthropathy (IMPA) is an important cause of joint disease in dogs. This condition occurs when immune complexes accumulate within the joint space, resulting in inflammation. Immune complexes are made up of antigens bound to antibodies. An antigen is a substance that the immune system recognizes as foreign and destroys. In contrast, antibodies are proteins made by white blood cells that bind to antigens, marking them for destruction and triggering an immune response. In some cases, the body recognizes its cells as foreign, creating an immune response against itself. The overall result of an immune response is inflammation and local tissue damage, which creates the hallmark joint swelling seen with IMPA.
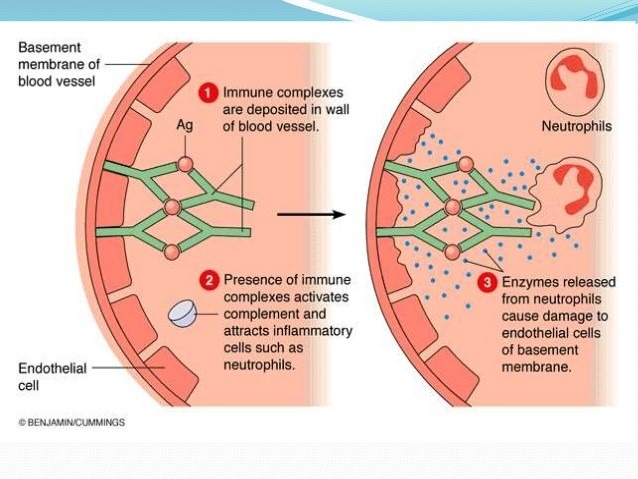
Figure 1: Formation of immune complexes and resulting immune response. (Karki, 2018)
IMPA is categorized based on the presence of deforming lesions. The erosive (deforming) type of IMPA causes damage to the joint cartilage and surrounding bony structures. Rheumatoid arthritis is the most significant form of erosive IMPA in dogs.2 The non-erosive form of IMPA is much more common and carries a more favorable prognosis. Nonerosive IMPA is further subdivided into the four types listed below.1,4
- Type I: no associated disease
- Type II: associated with infectious or inflammatory disease distant from the joints
- Type III: associated with gastrointestinal or liver disease
- Type IV: associated with neoplasia remote from the joints
Type I IMPA is primary or idiopathic IMPA, while Types II to IV are collectively called secondary or reactive IMPA. Primary IMPA is the most common form, accounting for about 50 to 65% of cases.2 A diagnosis is made once the underlying disease is ruled out. In Type II IMPA, an infectious or inflammatory process is identified at a site distant from the joints; it can be located anywhere in the body, such as the respiratory tract, heart, lower urinary tract, or reproductive tract. Infectious agents can be bacterial (e.g., tick-borne), viral, or fungal, and some inflammatory causes, such as pancreatitis, have been reported. Immune complexes are deposited into joint spaces due to the disease process, leading to an immune response and inflammation. Type III IMPA, or the enteropathic form, is related to gastrointestinal or liver disease. The gastrointestinal wall can become impaired with significant disease, allowing antigens to leak into circulation, accumulating immune complexes within joint spaces. Lastly, Type IV IMPA is associated with neoplasia (cancer) that is remote from the joints. Several forms of associated neoplasia include squamous cell carcinoma, lymphoma, and mammary carcinoma.2,4
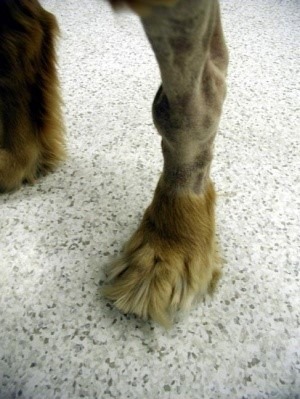
Figure 2: A swollen tarsus (ankle) in a dog with IMPA. (www.vetbook.org)
Other IMPA groups have been defined, including vaccine and drug-induced polyarthritis and breed-specific polyarthritis. Vaccine-induced IMPA can occur after a first vaccination or booster, and clinical signs develop within 30 days.4 Various drugs are associated with the development of IMPA, such antibiotics like penicillins and cephalosporins. Breed-specific IMPA is thought to have a genetic component as it affects certain breeds, including the Akita, boxer, beagle, Weimaraner, Bernese Mountain dog, Chinese shar pei, and spaniel breeds.2,6
Clinical Signs
Dogs with IMPA often present with clinical signs related to joint pain and decreased mobility. They have warm, swollen joints that are painful when touched. Any joint can be affected, and often multiple joints are involved. Dogs can be reluctant to walk or have a stiffened, stilted gait and appear to be “walking on eggshells.” Lameness can shift in different limbs, and signs of back or neck pain can be evident, too, such as a hunched posture or low neck carriage.6
Occasionally, signs of systemic illness are noted, and some dogs may present only with these signs. They are nonspecific and include lethargy, vomiting, weight loss, decreased appetite, and fever. Therefore, a lack of joint disease or altered mobility does not rule out IMPA as a differential diagnosis. In addition, IMPA is the most common cause of fever of unknown origin in dogs,1,4 so it should be considered in any case of fever without apparent cause.
Diagnosis
A clinical diagnosis of IMPA is achieved based on synovial (joint) fluid analysis. Arthrocentesis (joint tap) is when a needle is inserted into the joint space, and synovial fluid is removed. The procedure is typically performed under sedation or general anesthesia to allow for appropriate joint immobilization and to avoid the discomfort associated with the procedure. The fluid is submitted for cytology to evaluate its physical characteristics and cellularity. Multiple joints are sampled to increase the chances of finding cytological changes consistent with IMPA. A specific form of sterile inflammation, neutrophilic inflammation, in the synovial fluid confirms a diagnosis of IMPA. However, septic (infectious) inflammation can produce similar cytologic changes, so fluid samples are also cultured to better differentiate among these forms and rule out an infectious cause.3
A complete investigation for an underlying disease should be performed during the diagnostic process. Since there are various causative conditions, multiple diagnostic tests are often indicated as part of a thorough workup. Baseline tests include a complete blood count, chemistry profile, urinalysis, and urine culture. Additional diagnostics, such as chest radiographs or abdominal ultrasound, should be considered based on clinical signs or the results of initial tests. Radiographs of affected joints are also indicated to assess for erosive IMPA or other forms of joint disease. Lastly, infectious disease screening for tick-borne disease should be performed based on individual exposure risk and history.
Treatment
Treatment of IMPA varies based on the subtype, but the overall goals are to manage joint inflammation and to address any concurrent disease. Successful treatment of secondary IMPA depends on control of the underlying disease to remove the immune trigger. For example, drug-induced IMPA is treated by discontinuing the inciting drug, whereas IMPA secondary to tick-borne disease is treated with antibiotics. Joint pain and inflammation can sometimes be managed with analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs) alone. Immunosuppressive therapy is warranted in treating secondary IMPA if clinical remission is not achieved despite control of the underlying disease or if it cannot be treated.
Immunosuppressive therapy is the main treatment for primary IMPA,3 and it is started when concurrent disease is ruled out. Steroids (prednisone, prednisolone, methylprednisolone) are initially used and typically provide rapid clinical improvement. The steroid dose is carefully tapered once remission is achieved. Most patients with primary IMPA are on immunosuppressive therapy for several months from the start of treatment. However, some dogs may not attain remission with steroids alone or relapse while tapering the drug. In these instances, a second immunosuppressive agent further modulates the immune system and controls clinical signs. A second agent is also started if the side effects of steroids are severe, with the goal of lowering the steroid dose and reducing side effects.
Some patients can achieve complete remission, and all drug therapy can be gradually tapered and discontinued. Other patients require ongoing treatment with the lowest possible dose to maintain clinical remission, and treatment is lifelong in some dogs.
Prognosis
The prognosis of IMPA depends on the subtype, but it is generally fair to good. Primary IMPA carries a favorable prognosis, but relapse is possible during therapy or after completion. Secondary IMPA also has a good prognosis; dogs are less likely to relapse if the concurrent disease is identified and controlled. Other forms of IMPA, like vaccine or drug-induced, have an excellent prognosis.3
x
x
References
- Clements DN, Gear RNA, Tattersal J, Carmichael S, Bennett D. Type I immune-mediated polyarthritis in dogs: 39 cases (1997-2002). JAVMA. 2004; 224: 1323-1327
- Johnson K, Machkin A. Canine Immune-Mediated Polyarthritis Part 1: Pathophysiology. JAAHA. 2012; 48:12-17.
- Johnson K, Machkin A. Canine Immune-Mediated Polyarthritis Part 2: Diagnosis and Treatment. JAAHA. 2012; 48:71-82
- Rondeau MP, Walton RM, Bissett S, Drobatz KJ, Washabau RJ. Suppurative, Nonseptic Polyarthropathy in Dogs. J Vet Intern Med. 2005; 19:654-662
- Shaughnessy ML, Sample SJ, Abict C, Heaton C, Muir P. Clinical features and pathologic joint changes in dogs with erosive immune-mediated polyarthritis: 13 cases (2004-2012). JAVMA. 2016; 249: 1156-1164
- Wilson-Wamboldt J. Type I idiopathic non-erosive immune-mediated polyarthritis in a mixed-breed dog. Can Vet J. 2011; 52(2): 192–196.