-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Patty Ewing, DVM, MS, DACVP (Anatomic and Clinical Pathology)
By Patty Ewing, DVM, MS, DACVP (Anatomic and Clinical Pathology)![]()
angell.org/pathology
617-541-5014
Introduction
Image-guided, fine-needle aspiration (FNA) cytology of the liver can be a rewarding, practical and economical diagnostic tool for diagnosis of certain liver disorders. Moderate to highly cellular aspirates are readily obtained in many cases. Judicious use of this diagnostic tool is warranted because results can be incomplete or inaccurate in some instances. This article will review indications, limitations, contraindications and common cytologic findings of selected common liver disorders using FNA liver cytology.
Indications
Indications for performing liver cytology or incisional biopsy include persistently abnormal liver parameters, hepatomegaly, and abnormal echogenicity or masses noted on abdominal ultrasound (AUS). Liver cytology is often useful for the initial evaluation of hepatomegaly or mass lesions, but frequently requires incisional biopsy/histopathology for definitive diagnosis. Liver conditions that may be diagnosed via liver cytology include certain types of neoplasia (examples: hematopoietic neoplasia such as lymphoma, mast cell tumor, sarcoma and metastatic neoplasia), inflammation and infectious disease, hepatic lipidosis, and non-lipid hepatocellular vacuolar changes.
Limitations
Liver cytology does not allow for architectural assessment that is required for the diagnosis of certain liver abnormalities such as ductal plate malformation and circulatory disorders (e.g., portal vein hypoperfusion/microvascular dysplasia and congenital portosystemic shunts). Liver cytology may miss disease that is patchy, focal or multifocal in distribution such as inflammation or hepatocellular degeneration/necrosis. The zone of liver affected by inflammation, copper accumulation or degeneration/necrosis cannot be determined cytologically. Likewise, diagnosis of hepatic fibrosis, lobular atrophy or parenchymal collapse requires histopathologic assessment. Subtle features of canine chronic hepatitis are routinely missed by cytologic evaluation.
Correlating cytology results with biochemical findings may help overcome some of the limitations of liver cytology and better characterize the liver disease. For example, finding increased serum bilirubin and serum alkaline phosphatase (ALP) that is markedly higher than serum alanine aminotransferase (ALT) with increased numbers of inflammatory cells in the liver aspirate is supportive of cholangitis/cholangiohepatitis.
Cytologic evaluation cannot differentiate nodules or masses composed of hepatocytes including nodular hyperplasia, regenerative nodules, hepatocellular adenoma and well-differentiated hepatocellular carcinoma. Cystic lesions of the liver generally require histopathology for diagnosis given low cell yield or non-specific findings on cytology.
Contraindications
Primary contraindications of liver FNA include abnormal hemostasis and risk for clinically significant hemorrhage. A platelet count, assessment of platelet function (if warranted) and standard coagulation profile (aPTT and PT) are recommended precautionary tests. Inquiring about recent bleeding episodes, nonsteroidal anti-inflammatory drug (NSAID) use and anti-coagulant drug use is prudent. A cavitary mass or lesion (such as suspicion of a vascular tumor, septic abscess or risk of rupture) is a relative contraindication for liver FNA.
Normal Liver
FNA of liver typically produces a moderately cellular smear. Hepatocytes, frequently in clusters, represent the majority of nucleated cells obtained. Hepatocytes are 4-5 times the diameter of an RBC, polygonal in shape and have distinct cell margins. Nuclei are round, centrally located and have a single prominent nucleolus. Hepatocytes have abundant lightly basophilic cytoplasm with fine pink to blue granularity (see Figure 1). Occasion canine hepatocyte nuclei have rectangular inclusions, which are an incidental finding of no known diagnostic significance (see Figure 2). A low proportion of hepatocytes may be binucleated, have enlarged nuclei or cytoplasmic vacuolation. Small to moderate amounts of finely to coarsely granular green cytoplasmic pigment can be observed in normal hepatocytes (see Figure 2). Other cell types found in low numbers include endothelial or stromal cells, differentiating hematopoietic precursors and bile duct epithelium. Bile duct epithelium occurs in cohesive aggregates. The cells are of uniform size, noticeably smaller than hepatocytes, and have a small amount of pale basophilic cytoplasm and a round, regular nucleus (see Figure 1).
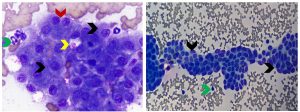
Figure 1. Aspirates from a normal canine (left) and feline (right) liver. Left panel: Majority of nucleated cells are large polygonal epithelial cells (hepatocytes) in clusters that have finely granular basophilic cytoplasm (black arrows). The green arrow identifies a neutrophil for size comparison. An occasional binucleated hepatocyte (red arrow) is a normal finding. The yellow arrow identifies amorphous pink material consistent with ultrasound transmission gel, a common contaminant in liver aspirates (Diff-Quik, 750x magnification). Right panel: Note flat sheets of uniform small polygonal to cuboidal epithelial cells arranged in tubular structures that are consistent with bile duct epithelium (black arrows). They are smaller than hepatocytes and have scant pale blue cytoplasm, a small round to oval nucleus, and dense, granular to homogeneous chromatin. The green arrow identifies a neutrophil for size comparison. (Diff-Quik, 500x magnification)
Pigments
Different types of pigment are commonly found in both normal and abnormal liver aspirates. Because most of the pigment is within the cytoplasm of hepatocytes and green in color in Wright-Giemsa- or Diff-Quik-stained cytologic specimens, special stains are usually required to definitively differentiate the various types. The most common pigment found in the cytoplasm of normal hepatocytes is lipofuscin, a “wear and tear” pigment associated with accumulated indigestible material within lysosomes. Large amounts of granular dark green pigment may be observed in hepatocytes of older dogs and cats (see figure 2). Accumulation of lipofuscin is considered part of the normal aging process and is not representative of disease. A modified Ziehl-Neelsen stain is used to confirm the presence of lipofuscin pigment. Bile accumulation in hepatocytes appears as variably-sized yellow to olive green or dark green to black pigment. Abundant amounts, especially when present as bile-filled canalicular plugs, are suggestive of cholestasis and may precede clinical icterus or hyperbilirubinemia. The plugs appear as distinctive intact and fragmented tubular accumulations of dark green, yellow or olive green pigment in cytologic specimens (see figure 3). A Hall’s bile stain may be useful for confirming the presence of bile (bilirubin). The cause of cholestasis, such as hepatic lipidosis or a neoplastic cell infiltrate, may be observed in cytologic specimens, although in many cases, the cause is not identified. Copper may be visible in liver aspirates stained with Diff-Quik or Wright-Giemsa as coarse, granular, pale blue, refractile pigment in hepatocytes, when present in large amounts although confirmation requires a special stain, such as rubeanic acid. Excess amounts of copper may cause hepatocyte injury or necrosis with inflammation. Copper accumulation can be a primary disorder such as the familial condition in Bedlington Terriers or a secondary finding in chronic hepatitis and other liver disorders. Hemosiderin appears as a granular golden-brown to blue-back pigment in the cytoplasm of hepatocytes stained with Diff-Quik- or Wright-Giemsa-stained cytologic specimens. Confirmation requires a special stain, Prussian blue. Hemosiderin is an iron-containing pigment that accumulates in hepatocytes during disease states such as hemolytic anemia, chronic inflammation, as well in patients that have received repeated blood transfusions or iron injections.

Figure 2. Aspirates from canine (left) and feline (right) liver. Left panel: Note granular dark green pigment in hepatocytes (yellow arrows) consistent with lipofuscin (normal “wear and tear” pigment). The black arrow identifies an intranuclear rectangular inclusion which is an incidental finding of no known clinical significance in the canine liver. Smaller amounts of pale blue, slightly refractile pigment (green arrows) found in hepatocytes was determined to be copper based on staining with rubeanic acid. (Diff-Quik, 1000x magnification) Right panel: Yellow arrows identify abundant granular dark green pigment consistent with lipofuscin in hepatocyte cytoplasm of a clinically normal aged cat. (Wright-Giemsa, 1000x magnification).
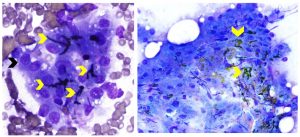
Figure 3. Aspirates from canine liver with cholestasis. Left panel: Note cluster of hepatocytes (black arrow) with interspersed dark green to black bile-filled canalicular plugs (yellow arrows). (Wright-Giemsa, 1000x magnification) Right panel: Yellow arrows identify several bile-filled canalicular plugs, yellow to olive-green in color, associated with a thick cluster of hepatocytes. (Diff-Quik, 500x magnification).
Hepatocellular Vacuolar Changes
The two most common types of hepatocellular vacuolar change, lipid and non-lipid, are shown in comparison to normal hepatocytes in Figure 4. Lipid vacuolar change in hepatocytes represents accumulation of triglycerides. The discrete clear (non-staining) vacuoles may be of variable size (very small to large). Lipid content can be confirmed with special stains including Sudan Black and Oil Red O. The most common clinical condition with marked hepatocellular lipid vacuolation is feline hepatic lipidosis, which may be primary (idiopathic) or secondary to underlying diseases such as pancreatitis, gastroenteritis, non-infectious or infectious cholangiohepatitis (see Figure 6) and neoplasia. Obese cats are predisposed to developing hepatic lipidosis. The patient history often includes a period of anorexia. Other possible causes of hepatocellular lipid vacuolation are shown in Table 1. Non-lipid vacuolar change typically results from accumulation of glycogen or water in the cytoplasm and appears as rarefaction or pale, feathery to indistinctly vacuolated cytoplasm. It is the most common type of hepatocellular vacuolation in the canine liver. Glycogen content can be confirmed with a special stain, periodic acid-Schiff (PAS). One of the most common types of glycogen accumulation is induced by exogenous or excessive endogenous corticosteroids (including hyperadrenocorticism), referred to as steroid hepatopathy. Marked elevation in serum ALP together with the finding of non-lipid hepatocellular vacuolar change is supportive of steroid hepatopathy. Other possible causes of non-lipid vacuolar change are shown in Table 1. Hepatocytes can exhibit a combination of lipid and non-lipid vacuolar change (see figure 7).
Table 1. Hepatocellular Vacuolar Change and Associated Conditions
| Vacuolar Type | Associated Conditions |
| Lipid | Idiopathic feline hepatic lipidosis Secondary feline lipidosis (anorexia, pancreatitis, gastroenteritis, hepatitis, neoplasia) Certain drug toxicities (example: canine aflatoxicosis) Lysosomal Storage Disease Juvenile hypoglycemia Diabetes mellitus |
| Non-lipid (glycogen or water) | Corticosteroid administration Endogenous corticosteroid excess (hyperadrenocorticism) Breed-specific progressive vacuolar hepatopathy (Scottish Terrier) Hyperaldosteronism Progestin excess Diabetes mellitus Insulinoma Hyperglucogonemia Metabolic disorders Copper chelation therapy Hepatotoxic insults Foci of nodular hyperplasia or regenerative hyperplasia Hepatocellular adenomas and carcinomas |
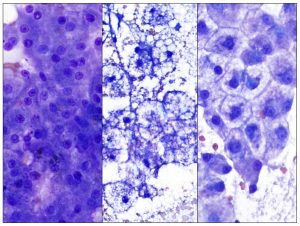
Figure 4. The panel shows normal hepatocytes (left), lipid-laden hepatocytes from a cat with hepatic lipidosis (middle) and cell swelling with indistinct vacuolation or rarefied cytoplasm in a dog with steroid hepatopathy (right). Diff-Quik stain, 1000x magnification.
Hepatic Inflammation
Evaluation of hepatic inflammation via cytology is typically incomplete because of the inability to assess inflammation relative to lobular architecture and because foci of inflammation may be missed in fine needle aspirates. Additionally, inflammation may be incorrectly diagnosed due to blood contamination with peripheral neutrophilia or lymphocytosis that creates the illusion of inflammation. Inflammation may be primary, reactive, clinically insignificant, multisystemic or secondary to inflammation at other locations such as the pancreas, gallbladder or gastrointestinal tract. Inflammation can be characterized as neutrophilic, lymphocytic, eosinophilic, mixed cell or granulomatous/pyogranulomatous. Finding inflammation in liver aspirates should always prompt a search for infectious agents (bacteria-see Figure 5, fungal or protozoa-see Figure 6). Finding pyogranulomatous inflammation or mixed cell inflammation in a feline liver aspirate may also prompt consideration of feline infectious peritonitis. Neutrophilic inflammation is suggested in aspirates with a high concentration of segmented neutrophils relative to the amount of peripheral blood contamination present or when clusters of neutrophils are associated with aggregates of hepatocytes (see Figure 5). A diligent search for bacteria is warranted, but they are infrequently found. Culture of bile may be worthwhile to evaluate for a bacterial cause when cholangiohepatitis is suspected. Lymphocytic-predominant hepatic inflammation is more commonly found in cats than dogs. Older healthy cats can have mild lymphocytic or lymphoplasmacytic inflammation restricted to portal tracts that are of no clinical significance. Moderate to marked numbers of lymphocytes found in feline liver aspirates may suggest lymphocytic cholangitis/cholangiohepatitis. Differentiation of lymphocytic cholangitis/cholangiohepatitis and small- to intermediate cell lymphoma can be nearly impossible based on cytology alone. Integration of all clinicopathologic findings, histopathology, and immunophenotyping +/- PARR (PCR for antigen receptor rearrangement) assay may be helpful in differentiating these two causes of hepatic lymphocytic infiltrates. Canine chronic hepatitis requires histopathology for diagnosis given the limitations of liver cytology. A wide range of inflammatory cells types (neutrophils, lymphocytes, plasma cells, macrophages and eosinophils) can be found in aspirates of canine chronic hepatitis. In some cases, inflammation is not detected due to patchy distribution, low numbers of inflammatory cells or fibrosis that leads to low cell yield.
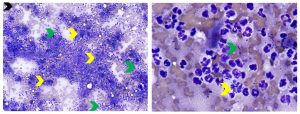
Figure 5. Aspirates of septic neutrophilic hepatitis in a dog. Left: Low magnification view showing marked increase in neutrophils (green arrows) relative to the amount of peripheral blood contamination present and clusters of neutrophils associated with degenerate hepatocytes (yellow arrows). (Diff-Quik, 200x magnification) Right: Higher magnification view showing degenerate neutrophils containing phagocytized bacterial rods (yellow arrows) and extracellular bacterial rods. (Diff-Quik, 1000x magnification)
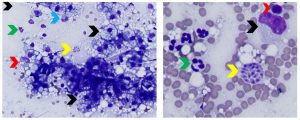
Figure 6. Aspirates of feline protozoal hepatitis and concurrent hepatic lipidosis. Left: Note mixed inflammation (neutrophils-green arrow, macrophages-red arrow), myeloid (neutrophilic) precursor shown by the blue arrow (extramedullary hematopoiesis), lipid-laden hepatocytes (black arrows) and a cluster of Toxoplasma gondii tachyzoites (yellow arrow). (Diff-Quik, 500x magnification) Right: Higher magnification view showing cluster of Toxoplasma gondii tachyzoites (yellow arrow), neutrophils (green arrow), a lymphocyte (red arrow) and a macrophage (black arrow). (Diff-Quik, 1000x magnification)
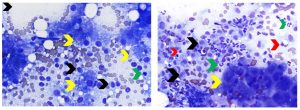
Figure 7. Aspirates of feline hepatic lymphocytic infiltrate and canine chronic hepatitis. Left: Note increased number of lymphocytes relative to the amount of peripheral blood contamination present. Lymphocytes are small (green arrow) to intermediate (black arrows) in size. Cytologic differentiation of feline lymphocytic hepatitis/cholangiohepatitis and small- to intermediate-cell lymphoma is often not possible, as in this case. Hepatocytes exhibit both lipid and non-lipid vacuolar change (yellow arrows). (Diff-Quik, 500x magnification) Right: Note cluster of hepatocytes (yellow arrow) adjacent to a large number of inflammatory cells that include neutrophils (green arrows), lymphocytes (red arrows) and macrophages (black arrows). Presumptive diagnosis of canine chronic hepatitis was made based on histopathology. (Diff-Quik, 500x magnification)
Extramedullary Hematopoiesis (EMH)
The adult feline and canine liver retains the ability to produce hematopoietic precursor cells. EMH is typically a non-specific finding in liver aspirates. The canine liver commonly develops EMH (especially granulopoiesis) in response to chronic hepatic disease due to a wide variety of causes. Late stages of erythroid precursors +/- granulocytic (see figure 6) and megakaryocytic precursors are commonly found in liver aspirates from anemic patients, especially when hemolysis or hemorrhage is the cause of the anemia. Hematopoietic precursors are also found with mature adipocytes in aspirates of myelolipomas.
Neoplasia and Hepatic Masses
Hepatocellular Masses
Masses composed of hepatocytes are commonly observed in the liver of middle-aged and older dogs and less commonly in older cats. Cytologic appearance of nodular hyperplasia, regenerative nodules, hepatocellular adenoma and well-differentiated overlap, thus histopathology is typically required for differentiation. Aspirates may be composed of normal appearing hepatocytes or hepatocytes exhibiting variable cytologic atypia. Some hepatocellular carcinomas exhibit sufficient cytologic atypia to warrant a presumptive diagnosis of carcinoma (see Figure 8).
Bile Duct-Associated Masses
Masses or cysts of bile duct origin occur more commonly in cats than dogs. Cholangiocellular carcinoma (CCC) is the most common primary hepatic neoplasm of cats. Feline congenital polycystic disease frequently involves the liver. Both of these entities can appear as many nodules (CCC) or cysts of variable size involving multiple liver lobes. Ultrasonographic appearance may be helpful in differentiating them. Aspirates of biliary cysts may yield mostly clear or pale yellow fluid and very few cells for cytologic evaluation. Aspirates of CCCs tend to yield more cellular aspirates. The epithelial cells obtained from either entity can be relatively uniform cuboidal epithelial cells with scant cytoplasm arranged in tubular/acinar formations or cohesive aggregates (see Figures 1 and 8), or densely packed sheets. Despite the well-differentiated cytologic appearance, bile duct carcinoma exhibits aggressive biological behavior and a high rate of metastasis. Canine cholangiocellular carcinomas may exhibit a greater degree of cytologic atypia, but often cannot be differentiated cytologically from metastatic ductal carcinomas.
Hemolymphatic Neoplasia
Lymphoma and hematopoietic neoplasia typically result in diffuse hepatomegaly (lymphoma or leukemia) and less often in one or more mass lesions (lymphoma). Large cell lymphoma involving in the liver of dogs is usually a component of multicentric lymphoma. Hepatosplenic lymphoma of dogs is a rare subtype of T-cell lymphoma. Lymphoma in the liver of cats can be either large cell multicentric lymphoma or small- to intermediate cell lymphoma (refer to discussion under Inflammation). Cytology is often a useful diagnostic tool for confirming the presence of large cell or high grade lymphoma in the liver if sufficiently cellular aspirates are obtained (see Figure 9). Acute leukemia (myeloid, lymphoid or other) can infiltrate the liver and produce aspirates with large numbers of immature hematopoietic cells types that may overlap in appearance with lymphoma cells. Flow cytometry and immunophenotyping may be useful in differentiating acute leukemia and lymphoma.
Mast cell neoplasia (mastocytosis) involving the liver generally represents visceral (hepatosplenic or GI) mast cell neoplasia in cats. In dogs, metastasis of cutaneous mast cell tumors can involve the liver. Low numbers of mast cells can be found in hepatitis and rarely normal livers; however, significantly increased numbers of mast cells, often in sheets or clusters, warrants diagnosis of mastocytosis. Cell morphology can vary from unremarkable to atypical (decreased cytoplasmic granulation and pleomorphism).
Disseminated histiocytic sarcoma is a biologically aggressive round (discrete) cell tumor that may involve the liver of dogs and less commonly, cats. Aspirates are often highly cellular and consist of pleomorphic mononuclear or multinucleated round cells exhibiting marked cytologic atypia and cytophagia. Immunocytochemistry (CD18 marker) may be useful in differentiating histiocytic sarcoma from other pleomorphic neoplasms. EMH is a common concurrent finding. A hemophagocytic variant of histiocytic sarcoma exists in dogs. Marked erythrophagocytosis is a common finding in this variant. Neoplastic histiocytes may appear well-differentiated and be difficult to differentiate from typical macrophages.
Plasma cell neoplasia is uncommonly found in liver aspirates, generally falls under the category of myeloma-related disorders, and occurs more commonly in feline than canine livers. Cytologic features include high numbers of plasma cells occurring in sheets or clusters. Plasma cells may be well-differentiated or exhibit a variable degree of cytologic atypia (see Figure 9).
Metastatic Epithelial and Mesenchymal Neoplasms
Liver is a common site of metastasis for many carcinomas and sarcomas. Liver metastasis is especially common for intestinal carcinomas and pancreatic carcinomas because of the vascular and lymphatic relationships. The tissue of origin cannot be determined cytologically for metastatic neoplasms to the liver in most cases (see Figure 10). Furthermore, differentiating metastatic carcinoma (especially ductal carcinomas) and primary cholangiocellular carcinomas based on cytology alone is very challenging or impossible. Cytology may be used for the oncology staging process or to prompt further evaluation to identify a primary neoplasm.
Primary sarcomas of the liver are far less common than metastatic sarcomas. Hemangiosarcoma is the most commonly observed spindle cell tumor of the canine liver. Neoplastic spindle cells must be differentiated from native /reactive stromal or endothelial cells and reparative fibroblasts, which can prove challenging in some cases. Moderate to marked cytologic atypia is useful in making a presumptive cytologic diagnosis of sarcoma (see Figure 10).
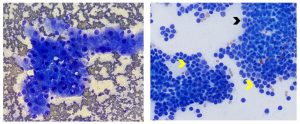
Figure 8. Aspirates from feline hepatocellular carcinoma (left) and cholangiocellular carcinoma (right). Left: Note cohesive aggregate of hepatocytes exhibiting cytologic atypia including increased nuclear to cytoplasmic ratio, moderate anisocytosis and anisokaryosis, one or multiple prominent nucleoli and multinucleation. Histopathology is required for definitive diagnosis. (Diff-Quik, 500x magnification) Right: Note epithelial cells that are smaller than hepatocytes and have a small amount of pale blue cytoplasm (black arrow) forming cohesive aggregates and vague acinar/tubular arrays (yellow arrows). The epithelial cells exhibit more variable cell and nuclear size than is observed in normal bile duct epithelium and the chromatin is coarsely reticular rather than smooth (see figure 1 for comparison to normal bile duct epithelium). (Wright-Giemsa, 500x magnification)
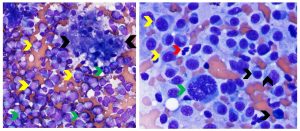
Figure 9. Canine hepatic lymphoma (left) and plasma cell neoplasia (right). Left: The yellow arrows identify numerous neoplastic, medium to large lymphoid cells with round or cleaved nuclei, finely granular chromatin and occasional bizarre mitotic figures (green arrows). A cluster of hepatocytes exhibiting vacuolar change is between the two black arrows. (Diff-Quik, 500x magnification) Right: Image shows high density of neoplastic plasma cells in a liver aspirate. Black arrows identify more well-differentiated plasma cells. Yellow arrows identify larger plasma cells exhibiting cytologic atypia. The green arrow identifies a very large binucleated plasma cell with coarse reticular nuclear chromatin. The red arrow identifies a neutrophil for size comparison. (Diff Quik, 750x magnification)
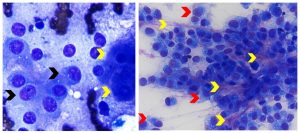
Figure 10. Aspirates of canine metastatic carcinoma (left) and metastatic osteosarcoma (right). Left: Note cohesive aggregate of neoplastic polygonal epithelial cells (black arrows) that are smaller in size than hepatocytes (yellow arrows) and have larger nuclei. They exhibit mild anisocytosis and anisokaryosis, and have multiple round to angular nucleoli of variable size. (Diff-Quik, 1000x magnification) Right: Note dense aggregates of neoplastic pyriform to spindle cells (red arrows) exhibiting moderate morphologic atypia (medium to large round nuclei, multiple round to irregular nucleoli, moderate anisocytosis and anisokaryosis). The yellow arrows identify intercellular pink smudgy matrix that correlated with osteoid matrix on histopathology. (Diff-Quik, 600x magnification)
Summary
Image-guided, fine-needle aspiration (FNA) cytology of the liver can be a rewarding, practical and economical diagnostic tool for diagnosis of certain liver disorders. Liver cytology is most useful for initial evaluation of diffuse hepatomegaly and hepatic mass lesions and is not useful for diagnosis of hepatic fibrosis, cystic lesions, ductal plate malformation and circulatory disorders such as congenital portosystemic shunts and portal vein hypoperfusion. Incisional liver biopsy and histopathology are often required for definitive diagnosis. Subtle features of canine chronic hepatitis are routinely missed by cytologic evaluation. Correlating cytology results with biochemical findings may help overcome some of the limitations of liver cytology and better characterize the liver disease.
References
- Valenciano, A and Cowell R: Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat, 5th St. Louis, MO: Elsevier, 2020, p.329-339.
- Raskin R and Meyer D: Canine and Feline Cytology—A Color Atlas and Interpretation Guide, 2nd St. Louis, MO: Saunders Elsevier, 2010, p. 226-247.
- Roth L. Comparison of liver cytology and biopsy diagnoses in dogs and cats: 56 cases. Vet Clin Pathol. 2001;30(1):35-38.
- Bahr KL, Sharkey LC, Murakami T, et al. Accuracy of US-guided FNA of focal liver lesions in dogs: 140 cases (2005-2008). J Am Anim Hosp Assoc. 2013;49(3):190-196.
- Wang KJ, Panciera DL, Al-Rukibat RK, et al. Accuracy of ultrasound-guided fine-needle aspiration of the liver and cytologic findings in dogs and cats: 97 cases (1990-2000). J Am Vet Med Assoc. 2004;224(1):75-78.
- Scott M, Buriko K. Characterization of the pigmented cytoplasmic granules common in canine hepatocytes. Vet Clin Pathol. 2005;34(suppl):281-282.
- Sepesy LM, Center SA, Randolph JF, et al. Vacuolar hepatopathy in dogs: 336 cases (1993-2005). J Am Vet Med Assoc. 2006;229(2):246-252.