-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Jennifer Michaels, DVM, DACVIM (Neurology)
By Jennifer Michaels, DVM, DACVIM (Neurology)
angell.org/neurology
neurology@angell.org
617-541-5140
Degenerative Myelopathy (DM) is a slowly progressive, degenerative disease affecting the spinal cord in older dogs. Degeneration begins in the axons then secondarily affects the myelin and the cells that produce it (oligodendrocytes) resulting in demyelination (Figure 1).
While definitive diagnosis of DM requires histopathologic evaluation of the spinal cord which is not possible ante-mortem, identifying cases where inclusion of DM as an appropriate differential is important for making appropriate diagnostic testing recommendations as well as providing information to owners regarding potential management options and prognostic information.
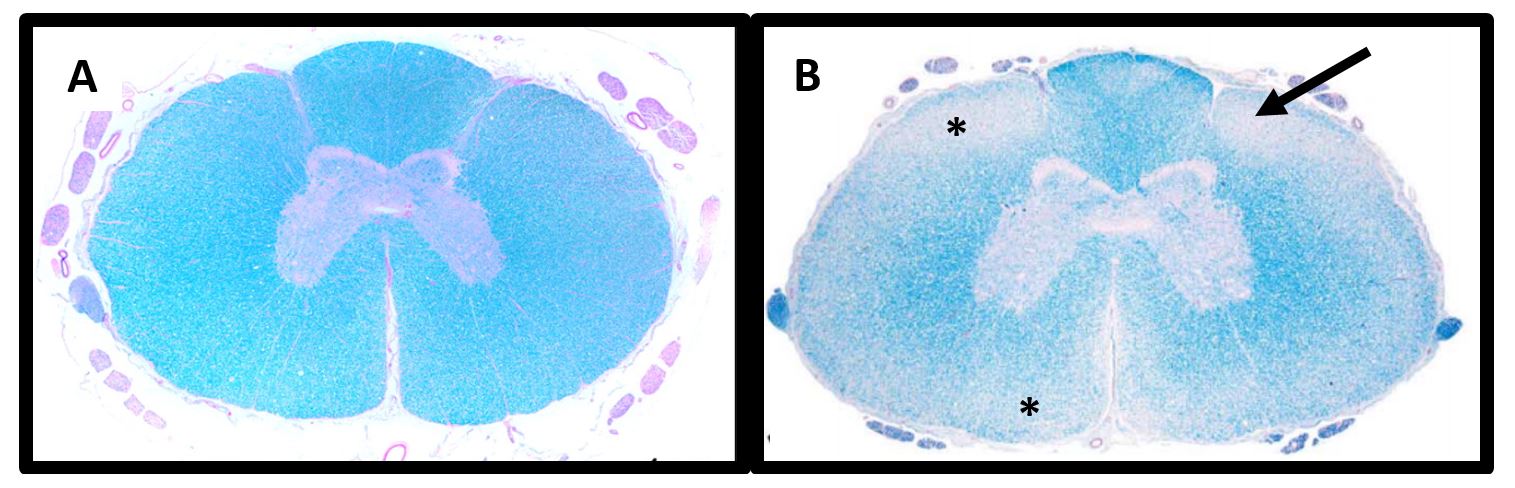
Figure 1. Spinal cord histopathology. A) Normal Dog: The white matter containing primarily axons and myelin is stained blue while the grey matter containing mostly neuronal cell bodies stains pink. B) DM-Affected Dog: Degeneration of the axons and myelin in the white matter is depicted by regions of palor (asterisk) which is classically most severe in the dorsal lateral funiculus (arrow). Images obtained from Awano T, et al. PNAS (2009). 106(8):2794-2799 and March PA, et al. Vet Path (2009). 46:241-250.
Signalment
DM affects older dogs with a vast majority of affected dogs being 8 years old or older. However, there have been infrequent reports of clinical DM in dogs as young as 5 years old. There is no sex predilection. It is a common misconception that DM affects only large breed or pure breed dogs. In fact, DM can occur in almost any breed, and has been reported in several small breed (e.g. Welsh corgi, pug, miniature poodle) and mixed breed dogs.
Prevalence of 0.19%.
Initial Clinical Signs
Classic clinical and historical features of degenerative myelopathy include:
- Slowly progressive clinical signs
- Paraparesis with an upper motor neuron (UMN) quality (i.e. spastic, long-strided gait with good muscle tone and normal pelvic limb spinal reflexes)
- Proprioceptive ataxia in the pelvic limbs (Figure 2)
- Lack of paraspinal hyperesthesia
These signs are consistent with a T3-L3 neuroanatomic localization. Clinical signs are often asymmetric.
While the classic T3-L3 myelopathy signs described above are the most common clinical presentation, about 10-15% of dogs with DM will initially present with lower motor neuron (LMN) signs in the pelvic limbs such as reduced patellar reflexes which is more suggestive of a L4-S2 myelopathy.
Disease Progression
Over time, the severity of the paraparesis and pelvic limb ataxia will progress such that the affected dog will become non-ambulatory and then paraplegic. At the point where motor function is minimal or lost entirely, patients will also develop urinary incontinence. With longer disease duration, patients will progress to LMN paralysis with loss of hind limb spinal reflexes and perineal reflex and development of LMN urinary and fecal incontinence. While eventually the disease will progress to involve the thoracic limbs, respiratory muscles resulting in hypoxemia and hypoventilation, and caudal brainstem resulting in dysphagia, it is extremely rare to see pets progress to these later stages as owners almost always elect for humane euthanasia prior to this time.
The duration of this progression is variable, but on average a large breed dog with DM will progress to non-ambulatory paraparesis over 6-9 months. In general, smaller breed dogs including Welsh Corgis are noted to have a slower progression.
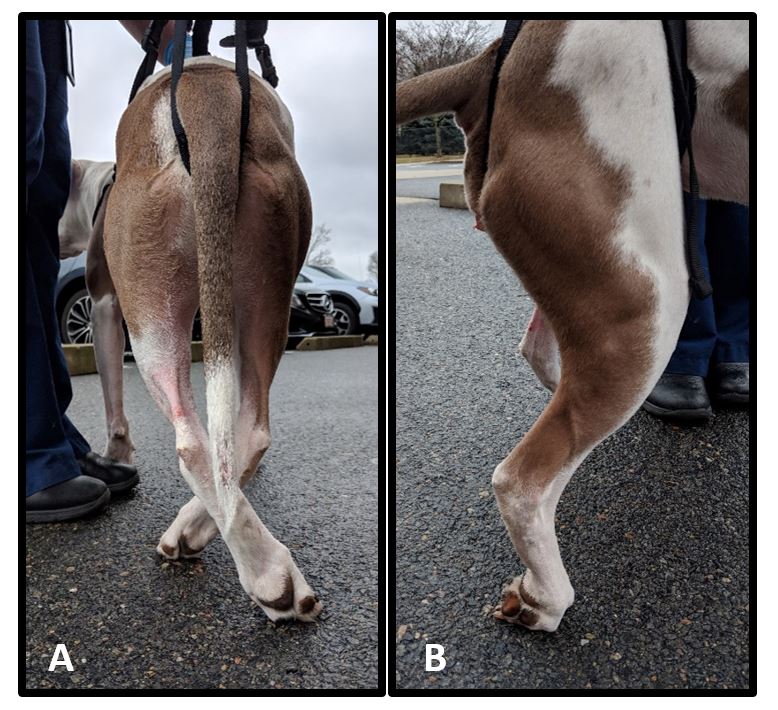
Figure 2. Pelvic limb proprioceptive deficits including crossing over (A) and knuckling (B). Proprioception is the sense of relative position of one’s own body. Proprioceptive abnormalities including ataxia and postural reaction deficits are signs of neurologic disease that would not be expected in patients affected by orthopedic disease alone.
Differential Diagnoses
Differentials for DM include other non-painful, slowly progressive T3-S2 spinal cord diseases including:
- Chronic intervertebral disc protrusions (Hansen type II)
- Degenerative lumbosacral stenosis
- Other degenerative causes of spinal canal stenosis (joint and ligament hypertrophy)
- Subarachnoid diverticulum (more common in some of the smaller breeds such as Pugs)
- Spinal cord neoplasia
The difficulty is that many DM patients will have concurrent neurologic (IVDD + DM) and orthopedic (DJD or hip dysplasia + DM) diseases. A thorough neurologic examination with findings including proprioceptive deficits should help distinguish between orthopedic and neurologic disease (Figure 2).
Genetics
Genome-wide association studies identified mutation in the SOD1 gene which codes for the superoxide dismutase 1 (SOD-1) protein. SOD-1 protein is abundant in the CNS and functions as a free-radical scavenger.
The primary mutation associated with the development of DM is very widespread having been identified in over 124 breeds including mixed breed dogs. Dogs can either have a homozygous normal N/N, heterozygous N/A, or homozygous mutated A/A genotype. An estimated 60% of dogs with the homozygous mutated genotype will develop clinical DM compared to approximately 5% of heterozygous or homozygous normal genotypes.
It is important to note that the finding of a homozygous mutated genotype does not predict with certainty the development of, nor definitively diagnose, clinical DM. Similarly, although the number is quite small, there are reports of dogs that developed clinical DM despite having a heterozygous or homozygous normal genotype. With the exception of the Bernese Mountain dogs (BMD) described below, full genetic analysis of the entire SOD1 gene in those dogs revealed no additional mutations to explain the clinical development of DM.
Interestingly, a second, breed-specific mutation associated with development of clinical DM has been identified in BMD. Some of the dogs that tested heterozygous or homozygous normal for the originally identified, widespread mutation were BMD that subsequently tested positive for this breed specific mutation.
Diagnostic Testing
Definitive diagnosis of DM can only be made histopathologically. Accurate ante-mortem diagnosis is based on recognition of the appropriate clinical picture, exclusion of other possible causes of the clinical signs, and documentation of SOD1 homozygosity.
Genetic testing is a critical part of ante-mortem diagnosis of DM. Testing for the widespread mutation is readily available. An at-home, cheek swab kit can be ordered by owners through OFA (www.ofa.org/dna/testing). The kit will be sent to the owner who collects the sample and mails it in. Alternatively, veterinarians can submit blood samples directly to the University of Missouri Animal Molecular Genetics Lab (http://www.caninegeneticdiseases.net/dm/sampledm.htm).
Results do need to be interpreted with care and in the context of the patient’s clinical signs. A result of homozygous normal (N/N) or heterozygous (N/A) indicate that it is extremely unlikely that the patient has DM or will develop it in the future. However, as indicated above, there are reports of normal or heterozygous dogs developing clinical DM. A result of homozygous affected (A/A) indicates that patient is at high risk for having or developing DM. It is important to remember, however, that not all at-risk dogs will develop the disease. For patients with clinical signs compatible with DM and who are genetically at-risk, additional diagnostics can be useful in ruling out other possible causes thereby lending support to the DM diagnosis.
Testing for the species-specific mutation in BMD is an important component of diagnostic testing in this breed, particularly if the clinical signs are consistent with DM but the affected dog is homozygous normal or heterozygous for the widespread mutation. The University of Missouri Animal Molecular Genetics Lab can perform this additional genetic testing.
DM causes no observable changes on MRI and cerebrospinal fluid analysis is often normal or shows no-specific changes; however, thoracolumbar MRI and spinal fluid analysis are still recommended to rule out other possible structural causes of T3-L3 myelopathy. If an owner declines MRI and CSF analysis, genetic testing can still be very useful to determine the pet’s risk for developing DM.
Therapeutic trials can also be a useful adjunct diagnostic tool. Steroids have no effect on clinical signs or progression of DM-affected dogs. In a patient where DM is suspected but cannot be distinguished from other differentials, a trial of anti-inflammatory steroids can help you decide if there is a structural cause of the clinical signs. For example, a patient with intervertebral disc protrusion(s) or spinal neoplasia may respond positively to steroids. If clinical improvement is seen with steroids, then you can confidently say that there is some other cause of clinical signs. However, it is important to recognize the limitation of this approach. If a patient responds to steroids suggesting some other etiology, this patient may still have DM and simply be concurrently affected by a steroid-responsive condition. Also, if a patient does not respond positively to steroids, this does not mean he/she has DM. Other etiologies such as disc disease or neoplasia don’t always respond positively to steroids.
Treatment
Unfortunately, there is no treatment currently shown to improve clinical signs or slow progression of disease in DM-affected dogs. Treatments that have been studied include: steroids, aminocaproic acid, vitamin B, C, and E, N-acetylcysteine, cobalamin, and tocopherol. No benefit was appreciated from any of these treatments.
One study documents an improved survival time in DM-affected patients treated with physiotherapy with survival time improving as the intensity of physical therapy increased.
| No physiotherapy | MST = 55 days |
| Moderate physiotherapy | MST = 130 days |
| Intensive physiotherapy | MST = 225 days |
Despite these promising results, there are limitations to this study which include lack of randomization, lack of definitive post-mortem histologic diagnosis, and owner bias. Still, physiotherapy is the only therapy to date that has shown even a potential benefit for DM-affected dogs, and it may improve quality of life for affected pets and their owners. Therefore, physiotherapy is strongly recommended for patients diagnosed with DM.
Prognosis
Long-term prognosis is grave as this is an invariably progressive disease; however, in the short term, many dogs can live a good quality of life while still ambulatory or even once they become non-ambulatory with the assistance of a cart. Survival time for an individual patient is highly dependent upon the owner’s willingness and comfort with caring for a severely neurologically affected dog. Owner education regarding expected progression of clinical signs including milestone changes (loss of independent ambulation, loss of voluntary urination, weakness in thoracic limbs, etc.) as well as necessary nursing and supportive care and tools for maximizing quality of life (e.g. physiotherapy, cart) is extremely important.
Survival times for smaller breed dogs tend to be longer (e.g. 20 months for Welsh Corgi vs. 11-16 months for Boxers and GSD); however, it’s difficult to know how much of this discrepancy is due to faster disease progression in the larger breed dogs or simply a reflection of the relative difficulty of caring for a large-breed dog compared to small-breed dog.
References:
- Coates JR, Wininger FA. Canine degenerative myelopathy. Vet Clin Small Anim. (2010). 40: 929-950.
- Kathmann I, Cizinauskas S, Doherr MG, et al. Daily controlled physiotherapy increases survival times in dogs with suspected degenerative myelopathy. J Vet Intern Med. (2006). 20: 927-932.
- Oyake K, Kobatake Y, Shibata S, et al. Changes in respiratory function in Pembroke Welsh corgi dogs with degenerative myelopathy. J Vet Med Sci. (2016). 78: 1323-1327.
- Wininger FA, Zeng R, Johnson GS, et al. Degenerative myelopathy in a Bernese Mountain dog with a novel SOD1 missense mutation. J Vet Intern Med. (2011). 25: 1166-1170.
- Zeng R, Coates JR, Johnson GC, et al. Breed distribution of SOD1 alleles previously associated with canine degenerative myelopathy. J Vet Intern Med. (2014). 28: 515-521.