-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Steven Tsai, DVM, DACVR
By Steven Tsai, DVM, DACVR
angell.org/diagnosticimaging
617-541-5139
diagnosticimaging@angell.org
The normal portal vascular system transports venous blood from the stomach, intestines, spleen, and pancreas to the hepatic sinusoids and ultimately the hepatic veins and caudal vena cava. Portosystemic shunting (PSS) occurs when abnormal vascular connections occur between the portal and systemic venous systems, bypassing the liver.1This can be acquired secondary to portal hypertension arising from chronic hepatopathy (such as cirrhosis) or congenital due to persistence of fetal shunting vessels. Imaging diagnosis of congenital shunts is important, since surgical attenuation is the treatment of choice in clinically affected patients.2
Portosystemic shunts can occur within the liver or outside of the liver. Intrahepatic PSS are more common in large breed dogs, while extrahepatic PSS are more common in small or toy breed dogs. Yorkshire terriers in particular are overrepresented with extrahepatic PSS. Cats can also present with both intrahepatic and extrahepatic PSS, although less commonly than dogs. Congenital PSS patients can present with a spectrum of clinical signs and labwork abnormalities. They are often smaller than their littermates, fail to gain weight, may show abnormal mentation generally or after a meal, may have chronic GI or urinary tract signs. Labwork abnormalities include microcytic nonregenerative anemia, hypoalbuminemia, decreased BUN, hypoglycemia, and hypocholesterolemia. Elevations in ALP and ALT are also common. Urinalysis may reveal ammonium biurate crystalluria. Liver function testing is routinely recommended in patients with suspected PSS, with elevated pre- and post-prandial serum bile acids being near 100% sensitive.1
An important differential diagnosis for dogs suspected of PSS is portal venous hypoplasia (PVH), also known as microvascular dysplasia (MVD). In this condition, there is direct communication between the (microscopic) portal venules and central hepatic veins, bypassing hepatic sinusoids.1 Clinical signs of dogs with PVH/MVD are indistinguishable from those in dogs with macroscopic PSS, and Yorkshire terriers are also predisposed. For this reason, definitive imaging diagnosis of macroscopic PSS is necessary to form an accurate clinical picture.
There are many imaging options available, with plain radiography being the only modality inadequate to make a diagnosis. Abdominal radiographs may show evidence of microhepatia and renomegaly, but no direct evidence of PSS is possible. Advanced imaging options include mesenteric portography, trans-splenic portography, trans-colonic portal scintigraphy, trans-splenic portal scintigraphy, nonenhanced ultrasonography, contrast-enhanced ultrasonography, CT angiography, and MR angiography. The three most common imaging studies performed at Angell for PSS are ultrasound, trans-splenic portal scintigraphy, and CT angiography.
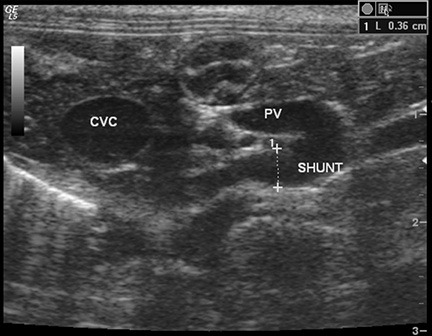
Figure 1 – Transverse ultrasound image at the level of the porta hepatis of a cat with a single extrahepatic PSS. A tortuous shunting vessel arises from the portal vein (PV), which is about half the diameter of the caudal vena cava (CVC).
Abdominal ultrasound is the most commonly performed imaging study in PSS patients. There are several advantages to ultrasound studies for diagnosing PSS. Ultrasound is relatively low to moderate in cost, especially compared to CT. Ultrasound evaluation does not require general anesthesia, although most patients will require moderate sedation. This is because the structures being imaged are small and difficult to visualize, and slight patient motion or abdominal tenseness can prevent full evaluation of the portal system. Ultrasound also does not require exposure to ionizing radiation. Ultrasound availability is generally more widespread than CT, MR, or nuclear scintigraphy. The primary drawback to ultrasound is variable sensitivity (74%-100%) and a high degree of dependence on operator skill/experience. A “shunt hunt” is not a routine component of an abdominal ultrasound, and a detailed technique is used to maximize detection of PSS. Typical secondary ultrasound findings in dogs with PSS include microhepatia, renomegaly, and urolithiasis. In one study of dogs evaluated for potential PSS, having all three of these findings on ultrasound was associated with a 100% positive predictive value for PSS.3 Patients with extrahepatic shunts typically will have a small portal vein at the porta hepatis, which is determined by comparing the diameter of the portal vein with the caudal vena cava or the aorta. Dogs with PSS typically have a PV:Ao ratio 0.8.3
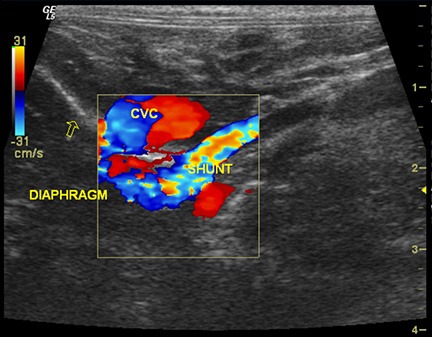
Figure 2 – Long axis view of the same cat as in Figure 1, cranial is to the left. Color Doppler imaging of the shunt indicates that it crosses the diaphragm (oblique line indicated by the open arrow) then turns and terminates on the CVC. This suggests a porto-phrenic shunt.
Further evidence of a shunt can be produced by injection of agitated saline into the spleen while sonographically assessing the caudal vena cava or right atrium.4Microbubbles in the saline are echogenic and may be visible in the CVC or RA in cases of PSS. Ideally, the actual aberrant vessel is visualized branching from the portal vein, typically at the level of insertion of the splenic vein or right gastric vein (Figure 1). Color Doppler interrogation of the vessel helps to indicate that blood is flowing from the portal vein into the aberrant vessel, rather than from the vessel into the portal vein as with normal tributaries. Visualization of the shunt termination is often possible if the termination is the CVC (most common), with turbulent blood flow noted in the CVC. The termination may not be visualized if the shunt goes to the azygos vein or phrenic vein, although with azygos shunts the aberrant vessel is often seen crossing the diaphragm dorsally, adjacent to the aorta (Figure 2). Intrahepatic shunts are reportedly easier to identify with ultrasound (95-100% sensitivity). Determination of whether the intrahepatic shunt is arising from the left, central, or right division of the portal vein is important, as well as whether the shunt is an actual aberrant vessel or a direct “window” connecting the PV with the CVC. This is important because intravascular coil embolization of a “window” type shunt is not possible.
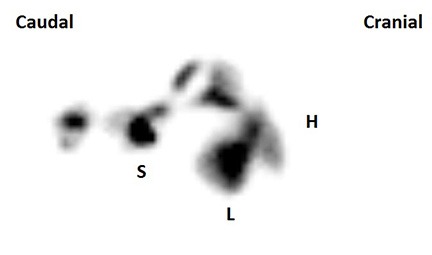
Figure 3 – Composite image of a trans-splenic portal scintigraphy in a dog without PSS. Radiopharmaceutical uptake is seen only at the site of injection in the spleen (S) and in the liver (L), with no activity noted at the level of the heart (H) or rest of the body.
If PSS is not identified on ultrasound, the next step is typically nuclear scintigraphy or CT angiography. The advantages of nuclear scintigraphy include low to moderate cost as well as a fairly definitive “yes” or “no” answer. Disadvantages include not very widespread availability, lack of precise morphologic information about the shunt, and the need to quarantine the patient for 24 hours due to radioactivity (Massachusetts law). The procedure requires a heavy plane of sedation (typically propofol titration), but usually takes only 5-10 minutes. A radiopharmaceutical (99mTc-mebrofenin) is injected into the spleen with ultrasound guidance, and dynamic images of the body are acquired for several minutes. Mebrofenin has a 97% first-pass clearance rate by the liver, so in a normal dog essentially all radiopharmaceutical uptake is seen in the liver alone, with little to no systemic activity (Figure 3). In dogs with a macroscopic shunt, radiopharmaceutical uptake bypasses the liver and enters the heart first, followed by the lungs, then systemic circulation, and gradually accumulates in the liver over time (Figure 4). In dogs with PVH/MVD, uptake is seen first in the liver, but subsequently is also seen in the heart, lungs, and systemic circulation. A shunt fraction can be calculated from the image data to determine the approximate percentage of portal blood bypassing the liver.5

Figure 4 – Composite image of a trans-splenic portal scintigraphy in a dog with PSS. Compared with Figure 3, note that radiopharmaceutical uptake is identified at the injection site (S), then coursing dorsally and cranially in a shunt vessel (*), bypassing the liver (L) and entering the heart (H). Note that early uptake is seen in the systemic circulation and kidneys (K).
CT angiography (CTA) has essentially supplanted mesenteric portography as the gold standard for diagnosing PSS in dogs. The primary advantage is precise morphologic information about the entire portal venous system and its relation with the systemic venous and arterial systems. Number and location of shunting vessel(s) can be determined to a degree not possible with ultrasound or scintigraphy. The disadvantages include high cost, need for general anesthesia, and lack of widespread availability of high speed multi-slice CT. Abdominal CTA also involves a fairly high dose of ionizing radiation to the patient. Abdominal CTA typically begins with a non-contrast scan of the abdomen to determine any other morphologic abnormalities. If there is concern for arterio-portal malformation, an arterial phase scan may be obtained by scanning the patient during contrast injection with a power injector. The portal phase scan typically commences following a 30-45 second delay, and a 3 minute delayed postcontrast scan finishes the study. When performed correctly, abdominal CTA provides very high quality images of the portal and systemic vasculature, and the study is subject to much less interpretive error than with ultrasound or scintigraphy6 (Figure 5).
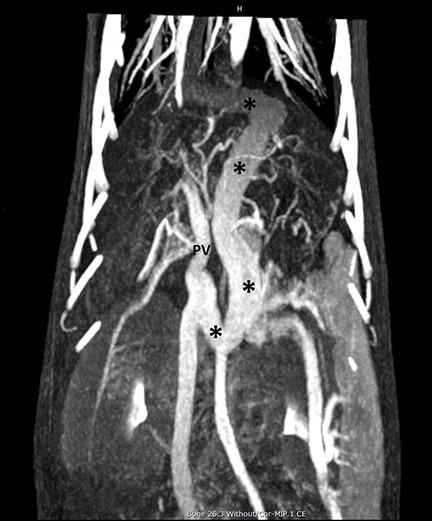
Figure 5 – Dorsal maximum intensity projection (MIP) of a CT angiogram in a dog with PSS. Cranial is at the top of the image, and the patient’s right side is on the left of the image. A large tortuous shunt vessel (*) arises from the portal vein (PV), courses caudally, to the left, cranially past the diaphragm, then turning rightward to terminate on the CVC. This is consistent with a left gastric-phrenic shunt. Note the generally excellent visualization of the abdominal and pulmonary vasculature.
In summary, portosystemic shunts are important congenital defects in dogs and cats which can be definitively diagnosed with imaging. Diagnostic workup and clinical management of PSS patients can be complex and is dependent somewhat on case specifics as well as the experience and preferences of the primary clinician, radiologist, and surgeon involved in the case. At Angell, ultrasound, scintigraphy, and CT angiography are most commonly performed in PSS patients.
For more information on Angell’s Diagnostic Imaging service or our online imaging consultative services, please visit www.angell.org/diagnosticimaging. Dr. Tsai can be reached at 617-541-5139 or via e-mail (diagnosticimaging@angell.org).
References
- Berent AC, Tobias KM. Portosystemic vascular anomalies. Vet Clin North Am Small Anim Pract. 2009 May 1;39:513–541.
- Greenhalgh SN, Dunning MD, Mckinley TJ, et al. Comparison of survival after surgical or medical treatment in dogs with a congenital portosystemic shunt. J Am Vet Med Assoc. 2010 Jun 1;236:1215–1220.
- D’Anjou M-A, Penninck DG, Cornejo L, Pibarot P. Ultrasonographic diagnosis of portosystemic shunting in dogs and cats. Veterinary Radiology & Ultrasound. 2004 Sep;45:424–437.
- Gómez-Ochoa P, Llabrés-Díaz F, Ruiz S, et al. Use of transsplenic injection of agitated saline and heparinized blood for the ultrasonographic diagnosis of macroscopic portosystemic shunts in dogs. Veterinary Radiology & Ultrasound. 2011 Jan;52:103–106.
- Daniel G. Scintigraphic diagnosis of portosystemic shunts. Vet Clin North Am Small Anim Pract. 2009 Jul 1;39:793–810.
- Nelson NC, Nelson LL. Anatomy of extrahepatic portosystemic shunts in dogs as determined by computed tomography angiography. Veterinary Radiology & Ultrasound. 2011 Sep;52:498–506.