-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 by Pamela Mouser, DVM, MS, DACVP
by Pamela Mouser, DVM, MS, DACVP
Anatomic Pathologist, Angell Animal Medical Center
pmouser@angell.org
www.angell.org/lab
617-541-5014
Current use of frozen sections at Angell
Following the acquisition of a new cryostat at the end of 2013 (nicknamed “Frosty Winters” and shown in figure 1), Angell pathology began offering intraoperative frozen sections on applicable cases. To date, use has been limited to intraoperative diagnosis and confirmation of a diagnostic sample. However, it is likely that the application of intraoperative frozen sections at Angell will progress to the evaluation of marginal tissue, which will further guide the surgical procedure. Frozen sections are most valuable when utilized in conjunction with other modalities (cytology, advanced imaging), as each procedure has its strengths and can complement the other techniques. This article provides background about this technique, and highlights some of its colorful history over the past 100+ years.

Figure 1: Cryostat currently in use at Angell. The microtome (denoted by the wide black arrow) is housed within the freezing chamber, which can be seen/accessed through the large sliding glass window at the center (delineated by thin white arrows). The chamber is kept at a temperature of about -20⁰C until the time of sectioning, at which time it is dropped to -40⁰C
Background
Frozen section technique was developed to address the need for rapid intraoperative pathologic diagnosis. While cytology from a fine needle aspirate (FNA) is commonly employed for the purpose of preoperative or intraoperative diagnosis, some pathologic processes are not amenable to FNA due to challenging sample location or poor exfoliation of cells within the lesion. In addition, cytology inherently lacks tissue architecture, which is needed to assess structural disruption of the tissue, spatial relationships between cell types, and invasive behavior of neoplasia. However, while cytology lacks tissue architecture, it often has finer cell detail as compared to frozen sections. Thus, cytology and frozen sections complement one another in the pursuit of an accurate and rapid intraoperative diagnosis.
The general process for creating a frozen section begins in surgery. A fresh tissue sample, which can be as small as a needle core or as large as a wedge biopsy, is immediately transferred to the laboratory while the patient remains under anesthesia. The tissue is embedded in specialized media (often OCT or “optimal cutting temperature” media) and placed into the freezing chamber of the cryostat, which is at an approximate temperature of -40⁰C. Once adequately frozen in a matter of minutes, the embedded sample is sectioned with a microtome housed within the freezing chamber (similar to sectioning of routine samples, but much colder!). Brief fixation in alcohol helps the sections adhere to the glass slide prior to rapid hematoxylin/eosin (HE) staining. The stained slide is coverslipped for microscopic review by the pathologist. A comparison of HE-stained frozen sections and routinely-processed formalin-fixed sections is demonstrated in figure 2.
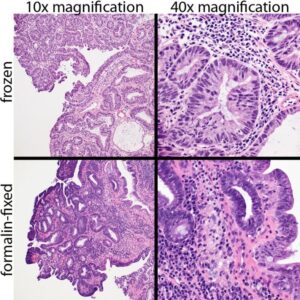
Figure 2: Canine rectal papillary adenoma, prepared using frozen section technique (upper two photomicrographs) and routine processing following formalin fixation (lower two images). Cellular detail is mildly reduced in frozen sections, but still adequate for diagnostic evaluation.
Intraoperative frozen sections in human medicine: A brief history
Almost 125 years ago, Dr. Thomas Cullen—a specialist in gynecological pathology at Johns Hopkins—published what is credited as the first written report on frozen sections.1 Individuals from other institutions and around the world were simultaneously working on frozen section technique. In 1905, just 10 years after Dr. Cullen’s publication, Dr. Louis Wilson of the Mayo Clinic detailed his own protocol for the rapid processing of frozen sections in the Journal of the American Medical Association.2 According to his report, Dr. Wilson could complete the entire process (from the freezing step to the time the sample was placed under the microscope for evaluation) in just 90 seconds. Compared to the typical 12-24 hour “routine” processing for formalin-fixed specimens, this type of turnaround time probably seemed nothing short of miraculous! Thereafter, the use of rapid frozen sections for intraoperative diagnosis exploded at the Mayo Clinic. In 1929, another pathologist from the Mayo Clinic reported on over 200,000 specimens processed as frozen sections,3 and touted the value—and also the beauty!—of this technique. Thereafter, articles focused on descriptions of method continued to accumulate in the literature, such as the seemingly humorous Russian paper entitled “Use of a home refrigerator as a cryostat”.4
As the procedure was increasingly utilized for diagnostic purposes, studies also began to evaluate the application and accuracy of frozen sections. A 1957 article from a two-part Canadian study showed that frozen sections were requested by surgeons to confirm one or more of the following: 1) diagnosis, 2) tissue identification, 3) margins/extent of disease, and/or 4) diagnostic quality of specimen.5 This information was then used to guide the surgical procedure and hopefully spare the patient from needing repeat surgery. In the 1957 study, a definitive diagnosis was deferred until formalin-fixed samples could be evaluated in 0.6% of cases, and a true difference occurred between the frozen section and “true” final diagnosis in 1.4% of cases.5 This is not drastically different from a 2013 Canadian publication which reported 3% deferred intraoperative diagnoses and 2% discordant diagnoses.6 Thus, while the error rate is low with intraoperative frozen section, it remains fairly consistent over many decades.
Use of intraoperative frozen sections in veterinary medicine
Intraoperative frozen sections are not commonly performed in veterinary medicine for several reasons. First, with the exception of teaching institutions and very few large specialty centers, most veterinary hospitals do not have an on-site histology laboratory to offer rapid processing of fresh tissue. Second, veterinary hospitals that do have laboratories may not pursue this technology due to the cost of equipment, required technical time/expertise, and insufficient clinical demand.
I found a single publication from 1993 discussing the use of frozen sections for intraoperative diagnosis in veterinary medicine.7 The authors evaluated a total of 194 frozen specimens from 172 animals—both dogs and cats—and compared the frozen section diagnoses to the “true” final diagnoses using formalin-fixed, paraffin-embedded tissue similar to the human studies referenced above. The study showed an overall accuracy of 93%, including 161 cases in which the frozen section diagnosis was essentially the same as the final diagnosis, and 19 cases in which the frozen section diagnosis identified the correct pathologic process (such as “inflammation” or “epithelial neoplasm”) and, when neoplastic, correctly determined benign or malignant. Twelve submissions (6%) had incorrect diagnoses. The incorrect diagnoses were split into errors related to sampling (insufficient tissue volume or inadequate lesional area, representing four cases) or interpretation (pathologist’s diagnostic judgment, making up eight cases). The final two cases (1%) had the diagnosis deferred until paraffin sections could be evaluated. The authors reported that this accuracy rate was similar to that which had been reported in human medicine at that time.7
A 6% error rate is admirable, especially given the wide spectrum of tissues and processes examined in the study. However, in contrast to human medicine, it is important to remember that a false positive diagnosis of malignancy (i.e. the diagnosis of a malignant neoplasm when there is not a malignancy present) in an animal could potentially result in the decision to euthanize prior to surgical recovery. Thus, while it is important to limit the number of deferred diagnoses and not negate the use of frozen sections, it is critical to clearly communicate a level of confidence along with the preliminary diagnosis to the surgeon at the time of frozen section.
We are excited to offer this service and look forward to expanding the use of intraoperative frozen sections to a greater diversity of surgical cases at Angell. This will be a useful tool in the diagnosis and intraoperative management of many conditions.
For more information, please contact Angell’s Pathology Service at 617-541-5014 or pathology@angell.org.
References
- Cullen TS. A rapid method of making permanent sections from frozen sections by the use of formalin. Johns Hopkins Hosp Bull 1895;6:67.
- Wilson LB. A method for the rapid preparation of fresh tissues for the microscope. J Am Med Assoc 1905;XLV:1737.
- MacCarty, WC. The diagnostic reliability of frozen sections. Am J Pathol 1929;5:377-80.
- Prokopchuk VS, Grechko II, Kurchenko IF, Chernobai IK. Use of a home refrigerator as a cryostat. Med Tekh 1974;2:54-5.
- Pitts HH, Sturdy JH, Coady CJ. Frozen sections. I. The effect on surgery. Can Med Assoc J 1957;77:943-7.
- Mahe E, Ara S, Bishara M, et al. Intraoperative pathology consultation: error, cause and impact. Can J Surg 2013;56:E13-18.
- Whitehair JG, Griffey SM, Olander HJ, et al. The accuracy of intraoperative diagnoses based on examination of frozen sections. Vet Surg 1993;22:255-9.