-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Maureen C. Carroll, DVM, DACVIM
By Maureen C. Carroll, DVM, DACVIM
angell.org/internalmedicine
internalmedicine@angell.org
617-541-5186
The Gut microbiome has emerged once again in the treatment of systemic disease, this time as it relates to disease of the liver. Various types of liver disease in people have been associated with alterations in the intestinal microbiota, so it may be fitting at this time to investigate the role of the microbiome as it relates to diseases of the liver in animals, both as a factor related to primary liver disease, as well as to bile acid metabolism.
The microbiome is a population of microorganisms that live in an established environment, and include bacteria, fungi, viruses, and parasites. The Intestinal microbiome includes one of the most-dense microbial populations on the planet ‑ 100 trillion microorganisms per average human. The microbiome is a factor in the maintenance of mucosal integrity and normal permeability of the intestinal mucosa.
Intestinal dysbiosis is a shift in the balance of gut microbes ‑ both in types of organisms and diversity ‑ adversely affecting the health of the host. Dysbiosis has been shown to be related to many diseases in human medicine including primary intestinal disease (i.e. ulcerative colitis), auto immune disease (i.e. multiple sclerosis), autism, metabolic syndrome/obesity, and more recently diseases of the liver and bile acid metabolism.
Blood flow to the liver is comprised of flow from the hepatic artery (which delivers oxygenated blood to the liver), as well as from the portal vein, which delivers deoxygenated blood from the intestinal tract, and accounts for 75% of the blood flow to the liver. It makes sense, therefore, that abnormal intestinal microflora would directly affect the liver as bacteria, bacterial by-products, cytokines, toxins, and metabolites are all delivered directly to the liver during first-pass metabolism.
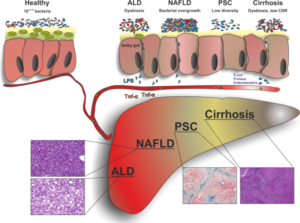
Various types of liver diseases in humans have been discovered to be related to intestinal dysbiosis, including primary sclerosing cholangitis (PSC), fatty liver disease (NAFLD), cirrhosis as well as alcoholic liver disease (ALD).
In NAFLD, patients often are identified with alterations in intestinal permeability, bacterial overgrowth of pathogenic species, and increased circulating levels of endotoxin and TNF α. The ‘microbiome signature’ in these patients appears to regulate liver and tissue fat storage, and is potentially a contributing factor to the pathogenesis of disease progression in these liver patients. PSC, also associated with a ‘microbiome signature,’ is commonly associated with people who suffer from inflammatory bowel disease. In these patients, there has been shown to be reduced bacterial diversity compared with control patients, as well as an increase in a species of bacteria, Veillonella. Furthermore, when nude mice undergo fecal transplantation with material from PSC patients, these animals actually show biochemical features consistent with PSC. Furthermore, Veillonella (part of the phylum Firmicutes) has also been associated with other chronic inflammatory disorders including fibrotic conditions. In cirrhotic patients, progressive abnormalities in the gut microbiota accompany disease decompensation associated with increased translocation of bacteria and increased circulating levels of bacterial DNA.
Based on the findings above, there is likely a role for prebiotics, probiotics as well as fecal microbiota transplantation (FMT) in the treatments of these human diseases, and potentially for the treatment of our veterinary patients. In addition, associated conditions with liver disease such as hepatic encephalopathy might also be associated with dysbiosis.
In different hepatic diseases, an impaired intestinal microbiota has been defined above. Increased gut permeability results in increased translocation of lipopolysaccharides (LPS) and inflammatory responses, TNF (tumor necrosis factor).
Diagram obtained/adapted from Clinical Liver Disease November 2016 Herbert Tilg M.D. , Christoph Grander M.D., Alexander R. Moschen M.D., Ph.D. Volume 8, Issue 5 November 2016 Pages 123–126 . Wiley.
To complicate the liver-dysbiosis relationship, once changes in liver integrity have occurred, the dysbiotic state can become progressively more deranged due to altered bile acid synthesis and metabolism.
Primary bile acids are synthesized by the liver and secreted into the intestinal lumen. Primary bile acids in dogs include Cholic and Chenodeoxycholic acid and are produced in the liver and conjugated to taurine or glycine to form bile salts before secretion into bile to assist in lipid digestion in the small intestine. Approximately 95% of secreted bile salts are reabsorbed from the small intestine via the enterohepatic circulation, the other 5% reaching the colon. Enzymatic reactions catalyzed by gut bacteria lead to the production of secondary bile acids, which include deoxycholic acid, urosdeoxycholic acid and lithocholic acid.
Studies have shown that in dogs with chronic enteropathy and dysbiosis, certain secondary bile acid concentrations are decreased. Abnormal fecal flora can result in an alteration in the process of hydrolysis of bile salts and conversion of primary bile acids to secondary bile acids. Because secondary bile acids can prevent germination of certain nefarious bacterial species in the colon, a reduction in these can result in bacterial overgrowth that results in a dysbiotic state. Conversely, increase in primary bile acid concentrations can actually promote the germination of unsavory species.
Furthermore, as we now know, the liver can be profoundly affected by intestinal dysbiosis and diminished intestinal microbial diversity. Not only does abnormal fecal flora result in reduced diversity and / or increases in pathogenic species, delivery of bacterial byproducts, metabolites and toxins to the liver; the affected liver then engages in bile acid synthesis, whose abnormal metabolism perpetuates the dysbiotic state.
FMT in humans has shown a reversal of fecal bile acid profiles as it relates to the treatment of clostridium difficile. The working theory is the correction of bile acid metabolism may be a mechanism by which fecal transplantation results in a cure in these patients.
Conclusion:
The intestinal microbiome is progressively becoming more and more relevant as it relates to the pathophysiology and treatment of diseases involving many body systems. Manipulation of the microbiota via FMT, or the use of pre or probiotics needs further investigation as these therapies related to liver inflammation, fibrosis, fat accumulation and other advanced liver diseases.
At Angell I have been performing fecal transplants in animals with GI disease for many years. Presently we are submitting Fecal Dysbiosis Indices on potential FMT patients with gastrointestinal disease (Texas A&M GI lab); we are performing FMT via enema (cats) or capsules (dogs). As we further investigate the role of the microflora as it pertains to systemic disease, the liver is next on our list of organ systems we will consider.
References
Clinical Liver Disease November 2016 Herbert Tilg M.D. , Christoph Grander M.D., Alexander R. Moschen M.D., Ph.D. Volume 8, Issue 5 November 2016 Pages 123–126 . Wiley.
Suchodolski JS. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet J Sep;215:30-7, 2016
AlShawaqfeh M, Wajid B, Guard M, Minamoto M, Lidbury JA, Steiner JM, Serpedin E, Suchodolski JS. A Dysbiosis Index to Assess Microbial Changes in Fecal Samples of Dogs with Chronic Enteropathy. J Vet Intern Med 30:4, p1536, 2016
Guard B, Toresson L, Honneffer JB, Blake A, Lawrence Y, Lidbury JA, Steiner JM, Suchodolski JS. Altered fecal bile acid metabolism in dogs with chronic enteropathy. J Vet Intern Med 30:4, p1455, 2016
Weingarden AR, Chen C, Bobr A, Yao D, Lu Y, Nelson VM, Sadowsky MJ, Khoruts A. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 15;306(4):G310-9, 2014
Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, therapeutics. Cell Mol Life Sci 65: 2461–2483, 2008
Giel JL, Sorg JA, Sonenshein AL, Zhu J. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLos One 5: e8740, 2010
Liver Disease and the Gut Microbiome – Medscape – Feb 03, 2017. Zetterman, Rowen K MD.