-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
Internal Medicine Service
Angell Animal Medical Center
617-541-5186
Megaesophagus (ME) is defined as a diffuse dilation of the esophagus resulting from decreased or absent motility. Esophageal motility is controlled by afferent neural pathways from receptors in the esophagus which are stimulated by the presence of food or water. Once stimulated, the impulses relay to the brain stem nuclei and swallowing center. Efferent motor pathways of the vagus nerve carry impulses to myoneuronal junctions in the esophagus. Although the pathogenesis of many forms of megaesophagus is not completely understood, failure along any of these pathways leads to dilation and clinical signs.
Although structural diseases, such as vascular ring anomalies, foreign bodies and strictures may cause esophageal dilation, these often result in localized dilation proximal to the obstruction. Megaesophagus is often divided into congenital versus acquired causes. Congenital causes are usually idiopathic in nature, where acquired may be primary (idiopathic), or secondary to other diseases such as myasthenia gravis, hypoadrenocorticism, canine dysautonomia, tetanus, polyradiculoneuritis, tick paralysis, lead toxicity, systemic lupus erythematosus, polymyositis and possibly hypothyroidism. Evidence for hypothyroidism as a secondary cause is low, however, there is one case report demonstrating a positive response to thyroid supplementation.
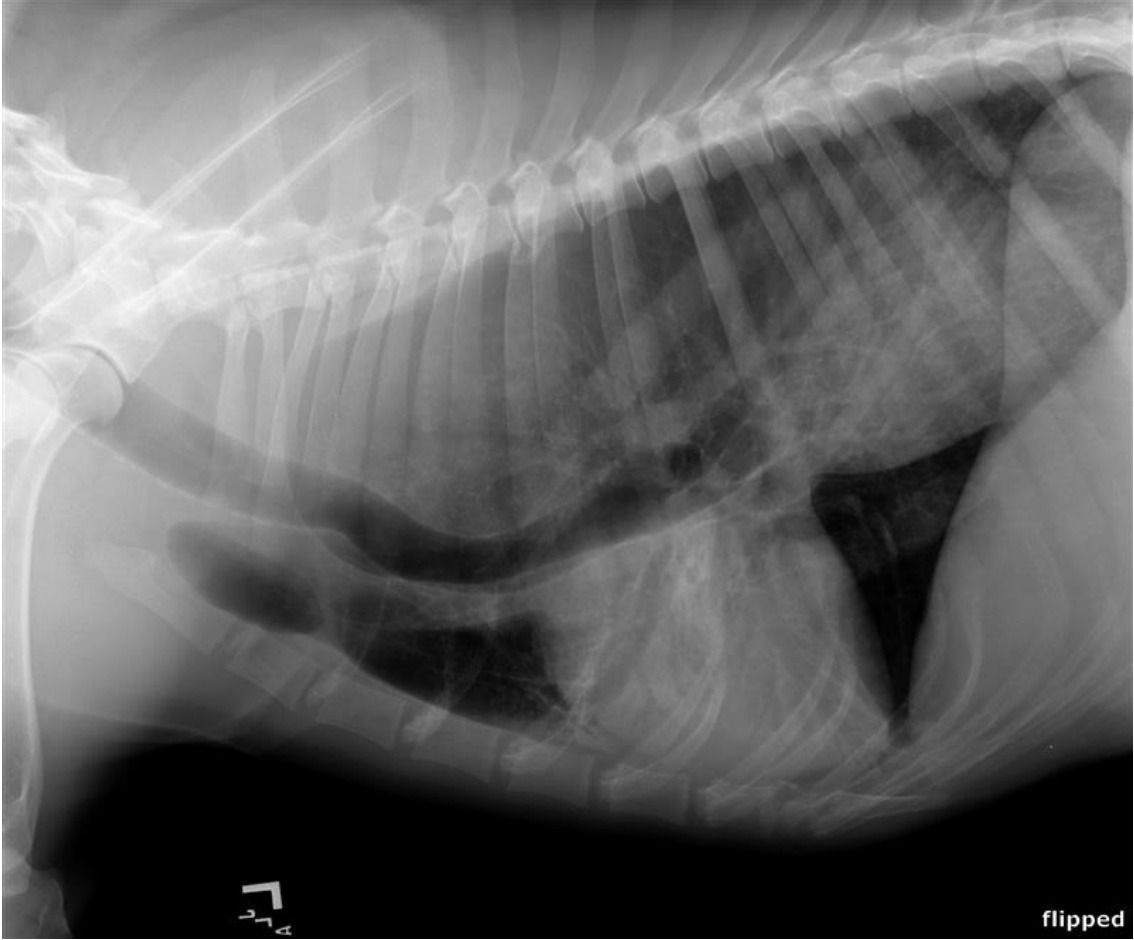
Figure 1: 8 year-old male castrated German shepherd diagnosed with megaesophagus at 6 months of age. Note the dilated, fluid-filled esophagus as well as air bronchograms in the right middle lung lobes, consistent with aspiration pneumonia.
Congenital ME has been documented in many breeds including the wire-haired fox terrier, miniature schnauzer, great Dane, Newfoundland, Rhodesian ridgeback and the Chinese Shar-pei. Clinical signs with congenital ME most often start shortly after weaning. With acquired ME, signs usually start when patients are middle age to older (7-15 years).
Patients often present with clinical signs of regurgitation which may occur shortly after eating to within hours of a meal. However, there is not always a time association with feeding noted. Other signs may include drooling, pain with swallowing, and anorexia from resulting esophagitis. Coughing, fever, lethargy, nasal discharge and/or difficulty breathing may be seen with concurrent aspiration pneumonia. Weight loss can be marked from chronic lack of nutrition. Signs referable to a secondary disease process may also be present.
Megaesophagus is diagnosed by persistent esophageal dilation on survey radiographs. Aerophagia and reflux esophagitis may result in temporary esophageal dilation. If signs resolve quickly or there is a reason to suspect a temporary condition, repeat radiographs are warranted before proceeding with a more advanced diagnostic work up. Positive contrast studies, videofluoroscopy and esophagoscopy are more often used to determine a cause with localized esophageal dilation or to diagnose altered esophageal motility in patients without radiographic evidence of megaesophagus.
Once testing has confirmed megaesophagus, evaluation for an underlying cause is recommended. Generally this would include a CBC, chemistry panel, urinalysis, blood lead level, creatine kinase, acetylcholine receptor antibody test, resting cortisol or ACTH stimulation and a thyroid panel. Testing can be prioritized based on any lab work changes or clinical signs that may indicate a more likely secondary cause.
Unfortunately there are no medical treatments that improve esophageal motility. Therapy is directed at any identified secondary causes, treatment of esophagitis, and supportive care, including nutrition and treatment of pneumonia. Feedings for patients should be small and frequent in nature with the patient in an elevated or upright position to take advantage of gravity for esophageal clearance. A Bailey’s chair may be feasible for some patients to assist with longer upright position times. Different diet consistencies (liquid, solids and chunks/meatballs) should be tried to determine what is best for an individual patient. Using a donut shaped e-collar or travel pillow at night may allow for additional elevation when resting and has been helpful for some patients at decreasing regurgitation. Calorie dense diets help to decrease the total volume needed daily, but the higher fat content may not be tolerated by all patients. Animals that cannot maintain adequate nutritional balance with oral feedings should be considered for a gastrostomy tube.
Even with diligent care, these patients are susceptible to aspiration pneumonia and this is a common reason for decreased quality of life with subsequent euthanasia. Although there is anesthetic risk with endotracheal or trans-tracheal washes, patients with repeated bouts of aspiration pneumonia would ideally undergo these procedures due to the concern for antibiotic resistance over time. Larger dogs with good temperaments often can do well with trans-tracheal washes using only light sedation and a local block. Intravenous or subcutaneous antibiotic options may need to be considered if patients are regurgitating and do not have a percutaneous gastrostomy tube. A recent case series describes the use of a wide-lumen, multi-side holed esophagostomy tube used to do periodic esophageal suctioning at home to reduce esophageal secretions. Although there were only 4 patients in this series, the number of episodes of regurgitation and aspiration pneumonia were significantly reduced so this may be considered for a select patient population.
A very recent social media posting from the University of Missouri Veterinary School has suggested that there may be a subpopulation of dogs with megaesophagus that have an achalasia-like (asynchronous) lower esophageal sphincter disorder. In this population, balloon dilation of the esophageal sphincter and/or Botox injections may allow sphincter relaxation and passage of food. More evaluation is needed for this concept but those that improve with esophageal sphincter relaxation may be candidates for a more permanent surgical procedure.
For patients with megaesophagus secondary to myasthenia gravis, therapeutic plasma exchange may be an option for treatment. Therapeutic plasma exchange (TPE), or apheresis, is an extracorporeal procedure that separates blood into its components such that plasma, and antibodies within the plasma, can be removed and exchanged with donor plasma. Though reports are limited, there are canine cases that presented with fulminant myasthenia gravis that responded to TPE, including resolution of megaesophagus.
The prognosis for patients with megaesophagus remains poor with a reported median survival time of 1-3 months after diagnosis. With diligent care and dedicated owners some will survive for extended periods. Hopefully with advancement in treatment options, we can continue to improve the quality of life and survival times for these patients.
For more information, please contact Angell’s Internal Medicine Service at 617-541-5186 or internalmedicine@angell.org.
References:
McBrearty AR, Ramsey IK, Courcier EA et al. Clinical Factors associated with death before discharge and overall survival times in dogs with generalized megaesophagus. JAVMA 2011; 238: 1622-1628.
Manning K, Birkenheuer AJ, Briley J et al. Intermittent at home suctioning of esophageal content for prevention of recurrent aspiration pneumonia in 4 dogs with megaesophagus. JVIM 2016; 30: 1725-1719.