-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
Angell Animal Medical Center, Dermatology Service
www.angell.org/dermatology
dermatology@angell.org
617-524-5733
Introduction
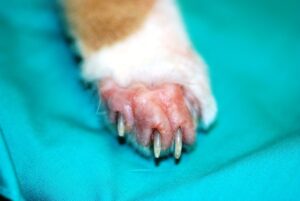
Image 1: Classic paraneoplastic alopecia with the “shiny” skin (also see image 4 below).
When dealing with patients suffering from a paraneoplasia syndrome (PNS) we deal with non-cancerous conditions associated with internal malignant cancerous condition(s). At least 30 different forms of PNS have been identified in human medicine. The best definition I know is: “Non-cancerous, neoplasm-related disorders that occur at a site distinct from the primary tumor or its metastases. They produce signs that reflect the remote effects of cancer rather than the direct effects caused by tumor growth or invasion.”
PNS is fairly well recognized in feline medicine and is typically seen more frequently in older cats without any breed or gender predisposition so far. Most of the symptoms associated with PNS are non-dermatological, for example anorexia, cachexia, fever, malaise/lethargy, polydipsia, and polyuria and hypercalcimia. All of these and more can be seen as a result of PNS, but obviously could be symptoms of other conditions (iatrogenic, physiological or non-cancerous pathogenesis).
Recognizing if symptoms are caused by PNS or not is an important part of our role in veterinary medicine, keeping in mind not all of our cancer patients develop PNS.

Image 2: Area of crusting and pruritus around the muzzle and rostral lip.
Since the dermatological symptoms associated with PNS can be easier to visualize than most internal changes, enhanced understanding of these clinical manifestations can be helpful for certain malignancies in our feline patients.
Several types of feline dermatological PNS have been identified. For example, Thymoma Associated Exfoliate Dermatosis (Rottenberg, von Tscharner et al 2004) and Dystrophic Calcification of the paws associated with pulmonary adenocarcinomas and a skin fragility syndrome in cats with adrenal tumors (Mauldin, Morris et al. 2002). Overall publications and literature are relatively minimal on these types of PNS with only a very small number of case reviews (Barrs, Martin et al. 1999, Turek 2003).
Patients with PNS are rare even in large tertiary referral hospitals like Angell. Here we will share case history suffering from Paraneoplastic Alopecia (PA) which is a rare PNS condition seen in older cats suffering from adenocarcinoma of the pancreas and/or the hepatobiliary system (Hall 2007, Florizoone 2008). The clinical findings are a particular presentation of alopecia with a glistening appearance of the skin. This symptom seen in Image 1 is considered pathognomonic for PA and is often described as “shiny skin” because it is as if the alopecic skin is coated in olive oil. This finding is due to complete loss of the skin’s top layer (stratum corneum) to the level of the stratum granulosum, the layer where the epidermal, fatty acids, cholesterols, and cholesterol esters are likely causing the oily shiny appearance.
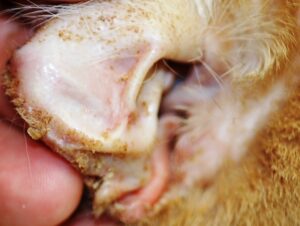
Image 3: Note the reflection of the light on the concave pinna along with the moderate serosanguineous crusting along the edge of the pinna
As expected, the cats suffering from neoplasia of the pancreas and/or hepatic system have bad prognoses with the exception of a few cases where a surgical resection of the tumors has had some life prolonging effect (Tasker 2008). Metastases to either liver or pulmonary tissue have been reported and in the literature have been suspected to precede the clinical dermatological symptoms.
Case History “Sara”
Sara was referred to the Angell Dermatology Service for investigation of a two week duration of progressive alopecia and weight loss with moderate to severe pruritus of the face. She was 10+-year-old indoor, spayed female orange and white domestic shorthaired cat. The owners had initially noticed crusting around edges of pinna, nares and mouth, no respiratory signs, an increased grooming and alopecia observed on the distal forelimbs, which progressed to the distal hind limbs. Routine hematology, serum biochemical profile and thyroid levels were performed prior to referral and showed no significant abnormalities. Sara was FiV/FeLV negative and there was no previous medical history except for routine annual vaccinations and intermittent ear infections, which would respond with unspecified topical therapy. She had no
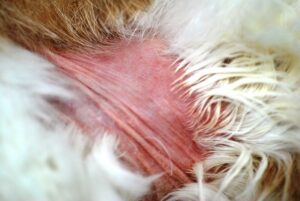
Image 4: Large area of alopecia on trunk, milder “shining” of the affected skin
history of travel outside New England and was fed a high quality commercial dry food diet. There was no history of skin disease among the humans in contact with the cat. Recent treatments included Clavamox 62.5mg, q12hrs and Tresaderm q12hrs for otitis externa. On examination the cat appeared slightly thin (BCS 2/5), weighing 3.18 kg, and her coat was unkempt. Bilaterally, symmetrical alopecia of all distal extremities, commissures of the mouth, nasal planum, and pinnae were apparent with focal areas of erythema and crusts with self-mutilation. The hair could easily be epilated from the margins of affected areas, leaving a striking shining appearance of the skin in the alopecic areas. The hair coat on dorsum was poor with mild seborrhea sicca, and footpads were soft with slight hyperkeratosis. The differential diagnoses for the alopecia included hyperadrenocorticism, malassezia dermatitis, dermatophytosis, superficial pyoderma, self-induced alopecia secondary to ectoparasites or allergy, demodicosis, immune mediated disease (pemphigus complex), paraneoplastic syndrome, and psychogenic alopecia.
Biochemical analysis was not repeated to contain expenses and since it was performed two weeks prior and was unremarkable. Skin scrapings and tape test were performed and proved to be unremarkable without evidence of ectoparasites or overgrowth of yeast. Trichograms and Wood’s lamp was negative and we found no obvious signs of ectothrix spores along the hair shafts to support the differential of dermatophytosis. Multiple skin 8 mm punch biopsies of the representing clinical lesions were performed under local anesthesia using buffered lidocaine (2% Bicarbonate 0.8%).
Histopathology
Findings from sampled sites revealed moderate to marked diffuse epidermal hyperplasia with mild parakeratosis and marked diffuse atrophy of the hair follicles, which lack hair shafts in follicular ostia/infundibulae. Clusters of secondary hair germ are often present at the base of atrophied follicles with multifocal sebaceous atrophy. Morphologically the haired skin demonstrated diffuse follicular atrophy with diffuse acanthosis and mild parakeratosis. The findings were consistent with the diagnosis of feline paraneoplastic alopecia. (Lee Gross 2008)
Ultrasonography was performed of the abdomen and demonstrated multiple variable sized hypoechoic nodules (<15mm) scattered in all lobes of the liver. Overall the liver was mildly enlarged and hypoechoic. Mild effusion was noted in the peritoneum. The pancreas was found to have an approximately 3cm round, hypoechoic mass with focal hyperechoic stippled areas. This mass was between the transverse colon and the stomach and ventral portal vein suggesting a pancreatic origin. Based on these findings, a fine needle aspirate of the pancreas was performed and sent in for cytology. The conclusion of the peritoneal effusion and multiple hypoechoic hepatic nodules and mass found in the left lobe of the pancreas was highly suggestive of a primary tumor consistent with a diagnosis of pancreatic adenocarcinoma.
Based on the guarded prognosis and worsening of clinical symptoms, the cat was euthanized. No necropsy was allowed.
Summary
Paraneoplastic alopecia is an uncommon condition with a grave prognosis with the exception of a few cases where a surgical resection of the tumors has had some life prolonging effect, but chemotherapy and/or radiation could have had a potential impact in these cases. To date very little has been published on these types of interventions. Most of the treatments we currently can offer are palliative and can reduce discomfort of the pruritus while managing secondary skin infections. The very small number of scattered cases makes clinical research of PNS difficult, and we need additional cooperative research between general practitioners, oncology, dermatology, radiology, internal medicine, clinical pathology, and surgery to investigate better detection, diagnostics and what kind of therapy could be considered for these cats.
For more information, please contact Angell’s Dermatology service at 617-524-5733 or dermatology@angell.org.
Literature
Rottenberg, C, von Tscharner, C. et al (2005) “Thymoma-associated Exfoliative Dermatitis in Cats.” Veterinary Pathology 41(4):429–433.
Barrs, V. R., P. Martin, et al. (1999). “What is your diagnosis? Feline paraneoplastic alopecia associated with pancreatic and bile duct carcinomas.” Journal Small Animal Practice 40(12): 595-596.
Godfrey, D. R. (1998) “A case of feline paraneoplastic alopecia
with secondarv Malassezia-associated dermatitis” Journal of Small Animal Practice (39);394-39.
Tasker, S. et al (2008)” Resolution of paraneoplastic alopecia following surgical removal of a pancreatic carcinoma in a cat”. 40(1)16-19 Journal Small Animal Practice
Mauldin, E. A., Morris, D. O. et al. (2002). “Retrospective study: the presence of Malassezia in feline skin biopies. A clinicopathological study.” Veterinary Dermatology 13(1): 7-14.
Turek, M. M. (2003). “Cutaneous paraneoplastic syndromes in dogs and cats: a review of the literature.” Veterinary Dermatology 14(6): 279-296.
Hall, J. (2007) “Diagnostic Dermatology.” Canadian Veterinary Journal 48(Feb) 203-204
Florizoone, K. (2008)”A case of feline paraneoplastic alopecia associated with
a pancreatic adenocarcinoma” Vlaams Diergeneeskundig Tijdschrift, 77:23-25.
Lee Gross, T et al (editors) “Chapter on diseases of the adenexa”; Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis, 2nd edition p 498-500.
