-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Daniela Ackley, DVM, DACVIM
By Daniela Ackley, DVM, DACVIM
![]() angell.org/internalmedicine
angell.org/internalmedicine
internalmedicine@angell.org
617-902-8400
January 2022
Introduction
In 2021, eight experts, including five internists, one radiologist, one clinical pathologist, and one anatomic pathologist, summarized current literature regarding the etiology, pathogenesis, diagnosis, and management of pancreatitis in the etiology pathogenesis, diagnosis, and management of pancreatitis cats1. The main goal was to offer clinically relevant suggestions for veterinarians based on evidence, and where such evidence was lacking, recommendations were based on the consensus of the experts in the field.
Pancreatitis in cats once thought rare, is a fairly common disease. In one study of 115 cats undergoing necropsy at UC Davis2, 66.1% had evidence of pancreatic inflammation. While acute lesions were found in 6.1% of cats, 50.4% had evidence of chronic pancreatitis, and 9.6% had both acute and chronic findings. However, 45% of healthy cats also had evidence of inflammation, suggesting the possibility of subclinical pancreatitis in many asymptomatic cats. Increased frequency of a diagnosis of pancreatitis over the last two decades is likely due to increased awareness and availability of minimally invasive diagnostics. Management of pancreatitis, however, remains challenging and definitive treatments are unavailable.
Definition
Acute pancreatitis is characterized by completely reversible inflammation after removal of the inciting cause, while chronic pancreatitis results in irreversible histopathologic changes. Clinically, however, it is impossible to distinguish acute pancreatitis from an exacerbation of chronic pancreatitis. Chronic cases commonly exhibit milder symptoms, while acute cases tend to be more severe.
Etiology
Pancreatitis has no age, sex, or breed predisposition, and no associations with body condition score, dietary indiscretion, or drugs have been established in cats. Infectious diseases rarely associated with feline pancreatitis include Toxoplasma gondii, coronavirus, parvovirus, herpesvirus, calicivirus, and certain parasites. Hypotension during surgery is likely a more critical risk factor in developing pancreatitis than surgical manipulation of the pancreas. Autoimmune pancreatitis occurs uncommonly in people, and there is some suspicion of immune-mediated etiology in cats, but the evidence is lacking. Pancreatitis in cats has been associated with several concurrent diseases, including diabetes mellitus, chronic enteropathies, hepatic lipidosis, cholangitis, nephritis, and immune-mediated hemolytic anemia (IMHA). Whether these conditions cause or are risk factors for pancreatitis is unknown.
In conclusion, 95% of cases of pancreatitis in cats are idiopathic with no specific etiology.
Pathophysiology
Premature activation of pancreatic digestive enzymes or zymogens (inactive forms of digestive enzymes) results in pancreatic autodigestion. The pancreas has several protective mechanisms to prevent this process (Pic. 1A). Hypotheses for spontaneous development of pancreatitis include activation of trypsinogen during biliary pancreatic reflux or duodenal obstruction, activation of zymogens by thrombin during bacterial toxemia, co-localization of lysosomal proteases and zymogen granules due to an apical block of zymogen granule secretion, or abnormal calcium signaling (Pic 1B). Researchers have concluded that trypsinogen activation is the initiating event for acute pancreatitis; however, the exact mechanism remains unknown. Recent hypotheses are suspected of abnormal calcium signaling and early activation of the nuclear factor kappa B pathway. Physiologic sequellae include an influx of neutrophils, increased vascular permeability, and loss of paracellular barriers.
The unique anatomy of the cat, with shared entry of the common bile duct and the pancreatic duct into the duodenum, may explain the association between acute bacterial cholangitis and pancreatitis.
In contrast to acute pancreatitis, trypsin activation is not the inciting cause of chronic pancreatitis. Cholecystokinin and oxidative stress likely alter calcium signaling and cause mitochondrial damage. Low-grade inflammation leads to the activation of stellate cells, which are the source of fibrosis. Ductal obstruction and irritation bypassing choleliths may be a significant factor, as suggested by a recent study from Cornell3 where 51% of cats with suspected idiopathic pancreatitis had passing choleliths documented by ultrasound or at the time of surgery. Some of them were microcholeliths, less than 3mm in size.
Clinical Signs
Clinical signs are nonspecific; 51-100% of cats show lethargy, 62-97% present with anorexia, only 35-52% have vomiting, and 11-38% have diarrhea.
Diagnostic Imaging
Abdominal radiography is neither sensitive nor specific for this disease. Recent studies established the normal and abnormal features of the feline pancreas imaged by multiphase contrast-enhanced computed tomography and magnetic resonance. Ultrasonography (US) remains the most used imaging modality and is considered part of the minimum database in these patients. Despite some limitations, US is important to detect comorbidities of the intestines, liver, and gallbladder. In cats, sonographic findings in acute pancreatitis may reveal pancreatic enlargement, decreased echogenicity, hyperechoic surrounding mesentery, and focal abdominal effusion (Pic 2). The sensitivity of these findings for diagnosing acute pancreatitis in cats is usually less than 67%, depending on the severity and operator’s experience. Features of chronic pancreatitis are even less defined and include hyperechoic or heteroechoic pancreas, a dilated common bile duct, pancreatic enlargement, and irregular margins (Pic. 3).
Ultrasound-guided fine-needle aspiration of feline pancreases was safe with diagnostic sampling obtained in 67% of cats.
Clinical Pathology
Chemistry findings are also variable. Abnormalities on CBC may show increased hematocrit due to dehydration and an inflammatory leukogram with a left shift. Increases in hepatic enzymes and bilirubin may be due to concurrent inflammation of the biliary tree, extrahepatic biliary obstruction, hepatic lipidosis, or some combination of these. In cats with severe pancreatitis, azotemia and a low urine specific gravity can result from an acute kidney injury secondary to hypoxemia, impaired renal microcirculation, or hypovolemia. Azotemia, hypocalcemia, and hypoglycemia have been associated with poor outcomes. Hypertriglyceridemia is not clinically significant in feline pancreatitis.
Measurement of pancreatic lipase to diagnose pancreatitis is complicated by the presence of many types of lipases in the body. DGGR-based assays (PSL by Antech) measure pancreatic lipase, hepatic and lipoprotein lipase, and even hemoglobin. Another measure of lipase is serum pancreatic lipase immunoreactivity (fPLI), which can be measured by a commercial ELISA test (Spec fPL). Most of the data suggest high sensitivity and specificity of this test in evaluating for the presence of pancreatitis. Sensitivity is higher for severe cases than for mild cases. A positive Spec fPLindicates pancreatitis, but a negative result does not entirely rule out this disease. SNAP fPL is a semiquantitative test that correlates well with the Spec fPL. Cats with a “normal” result are unlikely to have pancreatitis, while those with “abnormal” results might have pancreatitis or a Spec fPL in the equivocal range. Few studies showed discordance between Spec fPL and PSL.
Management of Acute Pancreatitis
The mortality rate in cats with acute pancreatitis has been reported to be between 9 and 41%, depending on the severity and associated comorbidities. Management is predominantly supportive and symptomatic and is extrapolated from humans and dogs. Identification of comorbidities (e.g., diabetes mellitus (DM), diabetic ketoacidosis (DKA), cholangitis, and chronic enteropathy) as well as management of complications (e.g., hepatic lipidosis, cholestasis, acute kidney injury, pneumonia, shock, myocarditis, disseminated intravascular coagulation (DIC), or multi-organ failure) play an important role in therapeutic success… There are no proven disease-specific treatments in humans that change the natural progression of acute pancreatitis, although the research is ongoing. A new leukocyte function-associated antigen 1 antagonist was recently approved in Japan in dogs.
Supportive therapy includes intravenous fluids because establishing normovolemia can limit tissue damage by improving pancreatic perfusion and oxygen delivery. Early, aggressive hydration with Lactated Ringers Solution (LRS) hastens clinical improvement in humans. Avoiding over-hydration is equally important as fluid overload is associated with increased morbidity and mortality. Anti-emetics are used to minimize fluid losses and reduce the risk of regurgitation and esophagitis. The most commonly used anti-emetics in cats are maropitant and ondansetron, which work by different mechanisms and can be combined. Metoclopramide has questionable anti-emetic effects but, when administered as a continuous rate infusion (CRI), increases gastric emptying and decreases gastric atony. Pain is challenging to evaluate in cats and is likely underestimated in cats with pancreatitis. Opioids should be used as the first line of analgesia; buprenorphine is adequate in most cats, while methadone or fentanyl can treat more severe pain. Maropitant was shown to offer some visceral analgesia and anti-inflammatory activity. Appetite stimulants play an essential role in preventing malnutrition and impairment of the gastrointestinal barrier secondary to anorexia. Mirtazapine is used most commonly, but capromorelin has been recently approved in stable cats with CKD. Capromorelin has been reported to cause hypotension and bradycardia in cats, so the use of this medication should only be reserved for use in non-hospitalized clinically stable cats.
Nutritional support plays a central role in managing acute pancreatitis in people and companion animals. Lack of enteral nutrition leads to impaired gastrointestinal motility, intestinal villous atrophy, compromised intestinal blood flow, altered barrier function, and disruption of the normal intestinal microbiota. Cats have high dietary protein requirements and a higher tolerance for dietary fat than dogs. Highly digestible diets, often labeled “gastrointestinal” diets, are recommended. Placement of a feeding tube (Pic. 4) is indicated for cats that fail to respond to appetite stimulants within 48 hours.
These cases are characterized by severe dehydration (often 8-10% dehydrated or more), failure to respond to medical management, hypotension, hypoglycemia, and ionized hypocalcemia. Severe complications include systemic inflammatory response syndrome, cardiovascular shock, DIC, pulmonary thromboembolism (PTE), or multi-organ failure. Cats with severe pancreatitis should be referred to specialty hospitals equipped to provide critical care support and monitoring.
Fresh frozen plasma is not recommended as a standard treatment in humans or cats and should be reserved for cats with coagulopathy. Antibiotics are not recommended for non-complicated cases of pancreatitis in cats unless a solid clinical indication or sepsis is present.
There is insufficient evidence to recommend glucocorticoids in cats with acute pancreatitis, as no studies have evaluated their use.
Dyspnea is a common complication of severe pancreatitis in cats with multifactorial origin (pleural effusion or pulmonary edema due to volume overload, acute lung injury, acute respiratory distress syndrome, congestive heart failure, pulmonary thromboembolism, or pain). Thoracic radiographs and echocardiography often permit rapid diagnosis and guide treatments.
Additional therapeutic strategies, including proton pump inhibitors, trypsin inhibitors, antisecretory agents, and antioxidants, have not been proven efficacious, and their use is not recommended. No clinical studies support the use of (hyperbaric oxygen therapy) HBOT.
Management of Chronic Pancreatitis
Little research is available on the clinical therapy of chronic pancreatitis in cats. Cats with chronic pancreatitis often have other diseases, and treatment of these conditions usually takes priority. However, if chronic pancreatitis is causing clinical symptoms and decreases the patient’s quality of life, it should also be managed. Traditional analgesics may not be effective for visceral pain mediated by cytokines, substance P, and neurokinin A. Acute exacerbation can be treated with buprenorphine; however, chronic pain may be better controlled with gabapentin, tramadol, and maropitant.
Dietary recommendations are controversial. The majority of the panel members did not have concerns about the dietary fat content in cats with chronic pancreatitis, given the lack of scientific evidence suggesting fat should be avoided. Anti-emetics and appetite stimulants should be used as needed, and, similar to treatment recommendations for the acute form of this disease, antibiotics are not indicated.
Prednisolone is a commonly used anti-inflammatory and immunosuppressive drug with potentially antifibrotic effects. Given the lack of scientific evidence to recommend its use, the risks should be weighed against the benefits for an individual patient. The most concerning side effects of steroid use in these patients are enhanced peripheral insulin resistance and diabetes mellitus.
Some experts use prednisolone only in cats that are not hyperglycemic and only at anti-inflammatory dosages (i.e., 0.5-1 mg/kg PO q24h on a tapering schedule). Other panel members use immunosuppressive dosages (e.g., 2 mg/kg q12h for five days, then 1mg/kg q12h for six weeks and taper after that) with close monitoring (i.e., clinical re-evaluation and measurement of fPLI after 2-3 weeks). If hyperglycemia develops or is pre-existing, cyclosporine can alternately be used (5mg/kg q24h for six weeks) with close monitoring (i.e., clinical re-evaluation and measurement of fPLI after 2-3 weeks).
If prednisolone or cyclosporine do not improve clinical signs and a reduction in fPLI, they should be discontinued. In cats that show improvement on anti-inflammatory/immunosuppressive treatment, continued therapy may be necessary. Cyclosporine has been reported to unmask latent toxoplasmosis, which needs to be considered in cats exposed to raw meat.
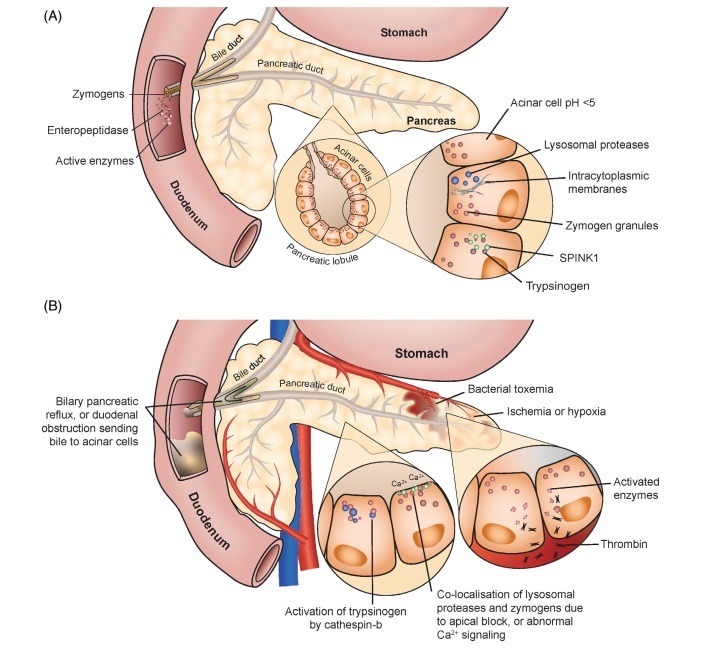
Pic. 1. Mechanisms protect the pancreas from premature activation of zymogens (A). Events can lead to acute pancreatitis due to the premature activation of trypsinogen (B).
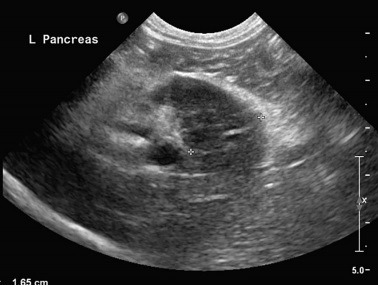
Pic. 2. Sagittal plane abdominal ultrasound image of the left pancreatic limb in a cat with acute pancreatitis showing enlargement, decreased echogenicity, and surrounding halo of hyperechoic mesentery.
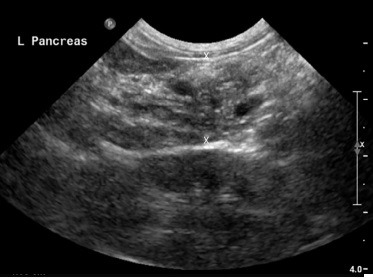
Pic. 3. Sagittal plane abdominal ultrasound image of the left pancreatic limb in a cat with chronic pancreatitis. The pancreas is mildly enlarged at 1.5cm (< 0.9cm), heterogeneous, and has mottled echotexture. The surrounding mesentery is unremarkable.
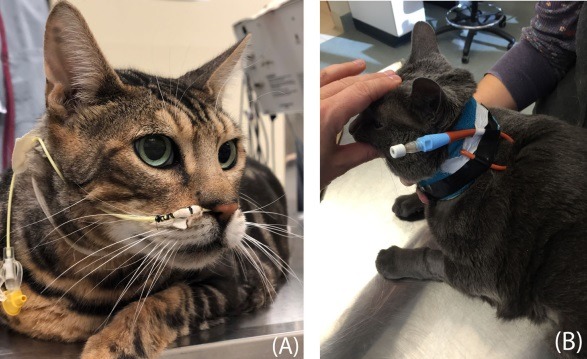
Pic. 4. Naso-esophageal (A) and esophagostomy (B) tubes are the most practical tubes for alimentary support of cats with acute pancreatitis.
References
- ACVIM consensus statement on pancreatitis in cats. Forman, Steiner et al. JVIM 2021; 35:703-723.
- Prevalence and histopathologic characteristics of pancreatitis in cats. De Cock, Forman et al. Vet Pathol 2007;44:39-49.
- Symptomatic cholelithiasis – A feline syndrome masquerading as “idiopathic pancreatitis.” ACVIM proceedings 2021:285-288.