-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Daniela Ackley, DVM, DACVIM
By Daniela Ackley, DVM, DACVIM
angell.org/internalmedicine
internalmedicine@angell.org
781-902-8400
MSPCA-Angell West, Waltham
Urinary bladder cancer in dogs is a challenging disease to diagnose, stage, and treat. Invasive transitional cell carcinoma (TCC) is the most common form of canine urinary bladder cancer affecting tens of thousands of dogs worldwide each year, and the prevalence appears to be on the rise. Most TCCs are intermediate to high-grade papillary infiltrative tumors; superficial, low-grade tumors are uncommon. TCC is most often located in the trigonal region of the bladder. In one large study of 102 dogs with bladder TCC, more than half of the patients had concurrent urethral involvement and about a third of male dogs had concurrent prostatic disease.1 Distant metastases are typically present in about 20% of cases at diagnosis, and are associated with a worse prognosis. More than 50% of dogs with TCC have distant metastases by the time of death2. Apart from regional lymph nodes and lungs, the metastatic spread can be seen in liver, spleen, kidneys, bones, adrenal glands, heart, brain, and skin.
The mean age at diagnosis is 9 to 11 years, with females being affected more commonly. A strong breed association has been reported with a 21-fold increased risk in Scottish Terriers, and a 3-6-fold increased risk in Eskimo dogs, Shetland Sheepdogs, West Highland White Terriers, Keeshonds, Samoyeds, and Beagles compared to mixed breed dogs.2 In dogs with high breed-associated risk, the sex predisposition is less pronounced. Environmental risk factors for developing TCC include exposure to older-generation flea control products, herbicides, and pesticides.3 Scottish Terriers that consumed vegetables at least three times a week had a 70% reduction in risk of developing TCC.4
The most common clinical signs include hematuria, dysuria, and pollakiuria. Dogs with the advanced disease resulting in ureteral obstruction and hydronephrosis may show signs of abdominal pain and have a palpable, enlarged kidney.
Diagnosis of a TCC is frequently delayed given the non-specific lower urinary tract signs. Many patients are treated with repeated antibiotic trials. While an antibiotic course may provide temporary relief of symptoms, the underlying tumor is progressing. Urinary tract infections (UTI) are common with 55% of dogs having at least one positive culture concurrently.5
Persistent or recurrent urinary signs despite appropriate antibiotic therapy warrant further investigation. In middle age to older dogs of breeds with high risk of TCC, it is appropriate to evaluate for TCC on initial presentation. Furthermore, blind cystocentesis is associated with risks of seeding the malignancy along the needle tract. The delay in correct diagnosis increases the possibility of metastatic disease by the time of detection, worsening the prognosis.
Abdominal ultrasound is commonly used to evaluate the bladder wall, kidneys and ureters, as well as for possible metastatic spread within the abdominal cavity. A recent study showed that some ultrasonographic characteristics including bladder wall invasion, echo pattern, and location can be used as prognostic indicators.6 In this study, dogs with ultrasonographic wall involvement (vs. noninvolvement), heterogeneous mass (vs. homogeneous mass), and trigone location (vs. other locations) had significantly shorter survival times. Ultrasound reliably predicted bladder wall involvement as confirmed by histopathology (Fig. 1). Definitive diagnosis requires cystoscopy or surgical biopsy of abnormal tissues with histopathology.
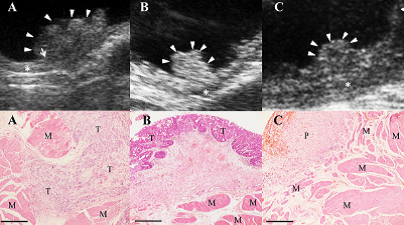
Fig. 1. Ultrasonographic images (upper panels) and corresponding histopathology slides (lower panels).
Picture A shows bladder mass invading the muscular layer (asterisk) as evidenced by histopathology (M smooth muscle, T tumor cells). There is no evidence of ultrasonographic or histopathologic invasion of deeper bladder layers in Pictures B (tumor) or Picture C (P polypoid cystitis). Hanazono KM, VRUS 2014;55(1):79-84.
New detection assay for TCC
Three recent articles published by researchers at North Carolina State University (NCSU) and the National Institutes of Health (NIH) describe detection of a new mutation in canine TCC tissue samples.7,8,9
These two independent groups of researchers discovered the same mutation in the same gene (BRAF gene), using two different approaches. Mutations of the BRAF gene are also found in human cancers and result in a mutated protein with increased kinase activity. This change leads to abnormal proliferation and tumor growth. Evaluation of hundreds of canine histopathologic TCC samples detected the BRAF mutation in 85% of them. These studies showed that the mutation was not present in non-neoplastic inflammatory bladder tissues, polyps, or in various other cancers. Researchers were able to develop a rapid and sensitive test to detect this mutation in cells shed into the urine called CADETSM BRAF Mutation Detection Assay. The data show that a positive result is >99% specific to TCC. That means that you can truly trust a positive result. However, the sensitivity is around 75-85%, so negative results do not rule out the possibility of dogs having BRAF-negative TCC. Another major advantage is that the assay is not affected by hematuria or bacteriuria. The test is also quantitative: the level of mutation generally correlates with the extent of disease. Higher levels of BRAF mutated cells are detected in dogs with advanced disease (visible mass on AUS). The test also detects dogs with emerging TCC not visualized yet by conventional methods such as AUS or cystoscopy. This test may be used in the future for monitoring of the response to therapy.
When a CADETSM BRAF Mutation test is negative, it could mean one of three possibilities: 1) The patient does not have a TCC, but instead has a benign polyp or inflammation; 2) the level of mutation is below the threshold of detection due to low number of cells shed into the urine, or 3) the patient is one of the 15% of dogs that has a TCC not associated with a BRAF mutation.
The laboratory requires 40mL of urine to be submitted, which can be challenging to collect in small dogs. This requirement applies to dogs that are asymptomatic and may have an early disease. Larger urine volume increases the chances of recovering sufficient cell numbers to detect BRAF mutation. However, if a dog is already diagnosed with a mass accompanied by urinary tract signs, 10-20ml is usually enough.
The company provides a preservative that stabilizes the urine once mixed, so urine can be collected from an individual dog on repeated occasions over a day or two to get the volume needed in the collection jar. The urine needs to be collected into a clean container and then immediately transferred to the collection jar, swirled to mix with the preservative and set aside at room temperature (out of direct sunlight) until it is sent to the laboratory.
Unfortunately, this test did not prove useful in cats. Only small numbers of cats were tested due to a low prevalence of TCC in cats; however, a BRAF mutation was not identified in feline TCC samples.
Future directions
Given the high sensitivity of this test and ability to detect very early disease, screening of middle-aged dogs from high-risk breeds can be considered. This approach was already put in practice, revealing the presence of the BRAF mutation more than 4 months before the onset of clinical signs. This provides exciting new opportunities for medical or surgical treatments, as complete surgical removal is not possible at later stages. Development of this new test holds promise to change our way of diagnosing and treating this insidious cancer in dogs.
References
- Knapp DW et al. Naturally-occurring canine transitional cell carcinoma of the urinary bladder: a relevant model of human invasive bladder cancer. Urol Oncol 2000;5:47-59.
- Knapp DW et al. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR Journal 2014;55:100-118.
- Glickman LT et al. Herbicide exposure and the risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. JAVMA 2004;224(8):1290-1297.
- Raghavan M et al. JAVMA 2004;225:389.
- Budreckis DM et al. Bacterial urinary tract infections associated with transitional cell carcinoma in dogs. JVIM 2015;29(3):828-833.
- Hanazono KM et al. Ultrasonographic findings related to prognosis in canine transitional cell carcinoma. VRUS 2014;55(1):79-84.
- Mochizuki H at al. Detection of BRAF mutation in urine DNA as a molecular diagnostic for canine urothelial and prostatic carcinoma. Plos One 2015:Dec:1-12.
- Mochizuki H at al. BRAF mutations in canine cancers. Plos One 2015:June:1-9.
- Decker B at al. Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder cancer – evidence for a relevant model system and urine-based diagnostic test. Mol Cancer Res 2015:13(6):993-1002.