-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 Pamela Mouser, DVM, MS, DACVP
Pamela Mouser, DVM, MS, DACVP
Anatomic Pathologist
pmouser@angell.org
angell.org/lab
617-541-5014
Introduction
In New England, where systemic fungal disease and other infectious lesions do not frequently cross the microscope stage, neoplastic conditions account for the majority of submissions. As with any pathologic process, the pathologist’s primary goal is to achieve a definitive diagnosis. In addition—particularly in the case of malignant neoplasia—the pathologist seeks to assess the extent of disease and provide a tumor grade in order to guide future treatment and offer prognostic information. Evaluation of inked surgical margins is a sometimes frustrating task fraught with various challenges, including tissue shrinkage and folding during processing, blurring or dilution of inked margins, and sliding of fascial planes to name a few. Tumor grading can be more objective, but depends on having established criteria for specific types of cancer. This article highlights what I consider appropriate application of grading schemes, including examples of grading systems I currently use for biopsy cases submitted to Angell.
The purpose of a histologic tumor grade is to predict biological behavior. In general, tumors categorized as “low-grade” or “grade I” are associated with a lower risk of recurrence, invasion, metastasis, and/or have a longer survival, while “high-grade” tumors have a greater risk of progression and/or death. A histologic grade should only be applied if objective, evidence-based criteria have been published for the affected species, tumor type, and lesion location, AND if the established criteria have been correlated with behavior. For example, a retrospective study evaluated histologic features (degree of differentiation and mitotic index) of digital squamous cell carcinomas relative to clinical outcome, and showed that histologic grade does not predict behavior, including new tumor development or metastasis.1 Thus, even though a tumor type might seem amenable to receiving a histologic grade (i.e. a well-differentiated squamous cell carcinoma with rare mitoses MUST be low-grade, right?), the application of such a grade is meaningless—or worse, inaccurate!—in the absence of studies correlating grade with prognosis.
There are a few common grading systems that I routinely apply to diagnostic cases, which I will summarize in the upcoming section. I encourage clinicians to alert pathologists to new publications on histologic grades, as articles may be published in a diverse array of journals not limited to pathology- or oncology-specific themes.
Canine cutaneous mast cell tumors
I began applying the new 2-tier grading system proposed by Kiupel et al. in 2011.3 In this system, the criteria evaluated by the pathologist are relatively objective, including a count of mitotic figures, multinucleated cells, cells with bizarre nuclei, and karyomegaly. If any one of these criteria surpasses the established cutoff value, the mast cell tumor is classified as high-grade. The study evaluated canine mast cell tumors of the skin; therefore, the criteria should not be applied to mucosal, visceral, or entirely subcutaneous/deeper soft tissue mast cell tumors (which were not evaluated in the study).
I find the emphasis on objective/quantifiable features to be a strength of the new 2-tier mast cell tumor grading system compared to the three-tier grading scheme published in 1984 by Patnaik et al.7 Criteria in the Patnaik system are established per grade. For example, a grade I mast cell tumor is confined to the dermis AND composed of round monomorphic cells AND includes medium-sized cytoplasmic granules AND lacks mitoses AND has minimal edema/necrosis.7 Since a grade II has its own combination of characteristics, how should a pathologist interpret a mass that has some features of each grade? When mast cells extend slightly into the superficial subcutis, I will diagnose a mast cell tumor as grade II, despite other features such as cell morphology and mitotic count best fitting a grade I diagnosis. Another pathologist may determine that the depth of extension is insufficient to warrant the increase in grade, as all other features fit the grade I classification. This lends a level of interpretation to the grading scheme, which in turn increases the inter-observer variation particularly in diagnosing grade I vs. grade II mast cell tumors with Patnaik criteria. Two goals of the newer 2-tier system are to decrease inter-observer variation of mast cell tumor grading and to more consistently predict highly aggressive mast cell tumors.3 In the 2011 study, a group of 28 veterinary pathologists evaluated mast cell tumors using Patnaik criteria, and the agreement among pathologists for diagnoses of Patnaik grades I and II was low, likely related to the reasons I have described above.3 Differentiating mast cell tumors as grade I vs. grade II using the Patnaik system was also determined not to be prognostically significant.3 Thus, one might argue that the new system simply lumps the two lowest Patnaik grades (I and II) into a single low-grade classification. While this seems true for a large proportion of cases, occasional tumors that would be categorized as grade II with the Patnaik system are actually classified as high-grade in the 2-tier scheme based on the additional morphologic features other than mitotic index (such as multinucleation, karyomegaly, etc). I would estimate that 90% of the cutaneous mast cell tumors I diagnose are classified as low-grade, with the remaining ~10% categorized as high-grade. Figure 1, shown below, compares a low- vs high-grade cutaneous mast cell tumor using the 2011 grading criteria.
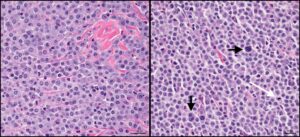
Figure 1: High magnification photomicrograph depicting a low-grade cutaneous mast cell tumor on the left and a high-grade on the right. Note the uniform appearance of mast cells in the left-hand panel, as compared to the variability apparent on the right. The vertical and horizontal black arrows depict multinucleated and karyomegalic cells, respectively, while the white arrow illustrates a mitotic figure. HE stain, 25 μm magnification bar.
Canine soft tissue sarcomas
To my knowledge, the most common grading scheme applied to canine soft tissue sarcomas (STSs) is based on a retrospective study published in 1997.4 The system assigns a score for each of three variables, which are: 1) the degree of differentiation, 2) mitoses per ten high power fields, and 3) proportion of necrosis in the examined area of tumor. Each variable receives a score from 1-3 and the sum of the three scores (anywhere from 3-9) determines the overall grade, which ranges from grade I (low) to grade III (high). Interestingly, a grading scheme outlining the same variables and scoring system had been published in 1984 for human STSs and presumably provided the basis for the 1997 veterinary publication.8 An important distinction between the two articles is that the total score could result in differing grades based on the published cutoffs. For example, a total score of 4 is considered a grade I by the 1997 veterinary system but would be a grade II based on the 1984 publication for human tumors.4,8 Soft tissue sarcoma grading based on these published criteria is limited to dogs with cutaneous/subcutaneous masses, and does not include tumors of the brachial plexus or oral/maxillary fibrosarcomas.
Some potential weaknesses of the STS grading systems above include “lumping” multiple histologic tumor types into a single group, using a subjective variable (degree of differentiation) as a scored component, and applying a score to necrosis. Tumor types included in the soft tissue sarcoma category include fibrosarcoma, peripheral nerve sheath tumor, myxosarcoma, liposarcoma, perivascular wall tumor (including hemangiopericytoma), pleomorphic sarcoma, malignant mesenchymoma, and undifferentiated sarcoma.2 Excluded from the STS group are histiocytic sarcoma, hemangiosarcoma, synovial cell sarcoma, leiomyosarcoma, and rhabdomyosarcoma. Some of these entities, such as perivascular wall tumors and peripheral nerve sheath tumors, may be difficult to distinguish histologically. Immunohistochemistry may assist in further phenotyping sarcomas, but is often not pursued due to client cost and availability. Therefore, a system of “lumping” is more realistic in classifying and grading canine STSs even if there may actually be behavioral differences among the various sarcoma types. Degree of differentiation is the most subjective and therefore the least reproducible variable of the STS grading system,2 which may lead to inter-observer variation in histologic grading. I consider necrosis to be a secondary effect of the tumor, and not an inherent characteristic of the neoplasm itself. While necrosis may be more common in rapidly-growing lesions that become hypoxic, external factors (such as self-trauma) may also induce tumor necrosis and cause an artificial increase in overall grade.
Canine and feline mammary carcinoma
Numerous published studies evaluate mammary carcinomas in domestic animals, as both dogs and cats have been proposed as animal models of the widely researched human disease. In a veterinary pathology textbook detailing neoplastic diseases of domestic animals, Misdorp outlines a three-tier grading scheme for mammary carcinomas in both species, which basically compiles multiple published findings.6 Each of three variables—level of tubule formation, mitoses/hyperchromatism, and nuclear pleomorphism—is scored from 1-3 and the sum of the scores determines histologic grade. In 2015, a novel grading scheme was proposed for cats which included lymphatic invasion, nuclear atypia, and mitoses.5 These variables were derived (and simplified) from a human breast cancer grading scheme published in 1991, and the three resultant grades show significant differences in survival. While this grading scheme is relatively new and based on a retrospective study, I appreciate the species-specific approach and the intent to make the grading criteria simple and objective for diagnostic purposes. I have begun providing both grades for feline mammary carcinomas as the two systems may differ. I am hopeful that follow-up studies will strengthen this proposed grading scheme.
Final discussion
There are several other grading systems for various canine and feline tumors that I have not described here and am unlikely to apply routinely to diagnostic cases. Advanced techniques, such as immunohistochemistry, proliferation markers, and prognostication panels, are supplemental tools that may eventually negate some of the older grading schemes that are based solely on histopathology. Currently, these ancillary tests are used in a supplementary fashion. For example, immunohistochemistry may be employed to further classify soft tissue sarcomas while the mast cell tumor prognostication panel provides additional prognostic data for canine cutaneous mast cell tumors.
Summary
It is important to reiterate that histologic grades are employed to PREDICT biological behavior, not to definitively proclaim the future. This has likely been experienced firsthand by those veterinarians reading this article who have treated dogs with “alleged” low-grade cutaneous mast cell tumors that have widely disseminated, or dogs with incompletely-excised high-grade soft tissue sarcomas that persist for many years without tumor recurrence. Tumor grading is certainly not a perfect science, which makes it all the more important to critically evaluate and appropriately apply grading schemes which are objective, evidence-based, and correlated with behavior.
For more information, please contact Angell’s Pathology Service at 617-541-5014 or pathology@angell.org.
References
- Belluco S, Brisebard E, Watrelot D, et al. Digital squamous cell carcinoma in dogs: epidemiological, histological, and immunohistochemical study. Vet Pathol 2013;50:1078-82.
- Dennis MM, McSporran KD, Bacon NJ, et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet Pathol 2011;48:73-84.
- Kiupel M, Webster JD, Bailey KL, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol 2011;48:147-155.
- Kuntz CA, Dernell WS, Powers BE, et al. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986-1996). J Am Vet Med Assoc 1997;211:1147-51.
- Mills SW, Musil KM, Davies JL, et al. Prognostic value of histologic grading for feline mammary carcinoma: a retrospective survival analysis. Vet Pathol 2015;52:238-249.
- Misdorp W. Tumors of the mammary gland. In: Meuten DJ ed. Tumors in domestic animals. 4th Ames, IA: Iowa State Press; 2002:575-606.
- Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol 1984;21:469-474.
- Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984;33:37-42.