-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
Twenty Questions with 20 Answers to Help Understand and Manage Uveitis
By Daniel Biros, DVM, DACVO
angell.org/eyes
617-541-5095
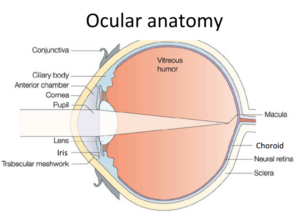
Uveitis is defined simply the inflammation in the “intra-”ocular, vascularized portion of the eye (the vascularized part of the eye within the inner border of the sclera excluding the retina). The uvea is further divided into three regions, the anterior portion (iris), the mid- portion (ciliary body), and the posterior portion (choroid) (Figure 1). From its most basic understanding any inflammation within the eye is dangerous since the visual axis cannot function if the media is cloudy from blood or vascular exudate/transudate or if the delicate tissue of the eye develops severe scarring and adhesions (synechiae). Further, any inflammation in the eye is associated with some degree of pain even if the inflammation is light relative to the condition present. In short, loss of vision and pain are the two most critical consequences of inflammation in the eye.
What gets more complicated, however, is in understanding the intricacies of uveitis including its triggers, how it commences, the difficulty at times of finding a specific etiology, and the challenges of treating-to-cure the condition without some residual damage or permanent change in the functioning eye. Blindness is the ultimate outcome to avoid. In addition, many cases of uveitis are accompanied by another eye disease and/or systemic disease, making the approach to treatment even more complex and multilayered. Nonetheless, despite extensive testing, the most careful monitoring, and our best efforts, intraocular inflammation at worst can progress to ultimate vision loss and defeat.
At best, when uveitis has passed, there is no apparent damage to the eye, and the uveitis is a one-off event with no recurrence. Some cases are easy to address, some are hard, and like a house fire, often you can avoid significant damage if you get to it early and ensure treatment is thorough and complete, responding to the severity of the condition with the appropriate course of action—and being prepared for recurrence or relapse.
The arc of uveitis generally has a common trajectory in general, with an acute phase, a convalescent or chronic (active) phase, and the post inflammatory phase. The goals of this article are to discuss in more detail about three important aspects of uveitis relative to the arc of disease; Recognition, Action, and Direction.
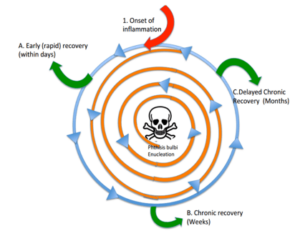
The illustration of all of the different pathways a course of uveitis can take is dizzying at worst, and informative at best, for understanding where in the course of disease a patient may be at any given time (Figure 2). The inciting event or trigger is at the top of the figure (red arrow) and the course of inflammation proceeds in a circular counter-clockwise direction. The center of the diagram depicts blindness, or end-stage inflammation of the eye (defeat), otherwise known as phthisis bulbi, which is nature’s way of handling severe eye inflammation short of enucleation, which can be the other outcome at the center.
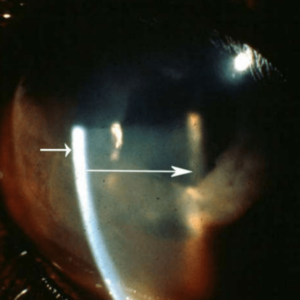
Photo B: Aqueous Flare (corneal border at end of short arrow, lens surface boarder at end of long arrow)
Chronic uveitis can spiral (orange line) from the outermost border of the diagram towards the center, with or without treatment, that is if the treatment and immune system cannot stop the inflammatory process. If the uveitis resolved in the acute phase, the trajectory heads (green arrow) out of the inward spiral and the condition is for now quiescent. When the uveitis passes into a more chronic phase the arc of disease continues in a circle and either tracks to a chronic phase which can be an ongoing cycle managed with long-term treatment to control, with observation, or alternatively the uveitis course could at any time spiral inward (progression towards phthisis and vision loss) or outward (progression towards recovery, or quiescence). While this is not the only way to characterize the course of uveitis, we demonstrate here the potential for inflammatory eye disease to be cyclical, and in a closed organ system such as the eye, where the only ins and outs are largely through the limited vasculature and lymphatics, it can be more difficult to eradicate the inflammatory process since the eye can act as a reservoir for inflammatory mediators once the inflammation ramps up.
So let’s ask some questions that help us navigate the circuitous course of uveitis. We’ve coordinated some of the very common questions ophthalmologists will ask themselves when treating uveitis, arranged in to the three areas mentioned earlier: Recognition, Action, Direction.
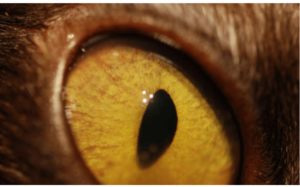
R1: What are the parts of the uvea and why is this important to know when testing uveitis? What are the common medical terms used to characterize aspects of uveitis?
The anterior uvea consists of the iris and ciliary body (Figure 1). The posterior uvea consists of the choroid. Anterior uveitis is inflammation of the iris and ciliary body typically (iridocyclitis), although it is possible to have iritis and cyclitis separately. Posterior uveitis is limited to the choroid (Figure 1). Further choroiral inflammation combined with retinal inflammation is termed chorioretinitis. Finally, the term panuveitis usually refers to inflammation in all segments of the uvea. Panophthalmitis is a term used to suggest inflammation in all parts of the eye including the uvea. Ciliary muscle spasm accompanies cyclitis and is one of the primary causes of pain in uveitis, and manifest as miosis (Photo A). Atropine paralyzes the ciliary muscle when applied topically to the eye and alleviates the pain due to the cramping of this muscle. Pupil dilation is a sign the medication is working. Anterior uveitis can produce aqueous flare (Photo B), which is manifest by protein accumulation in the anterior chamber. The aqueous humor becomes cloudy and if severe enough the view to the posterior segment and even the lens becomes difficult. Posterior uveitis (choroiditis or chorioretinitis) can manifest as vision impairment or blindness depending on the extent of the process (Photo C). Pressure fluctuations can occur in uveitis and typically result in a pressure drop relative to the unaffected eye. In acute uveitis or in cases of significant inflammation in the iridocorneal angle, the intraocular pressure may be elevated.
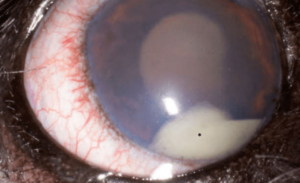
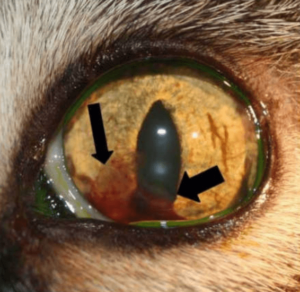
A few other important terms are used to characterize uveitis. Hypopyon (Photo D, asterisk) refers to the accumulation of inflammatory cells in the anterior chamber leading to the appearance of white precipitate in the inferior anterior chamber. Keratic precipitates (Photo E, arrows) are a subset of visible hypopyon that stick to the posterior cornea as white or grey aggregates or spots typically in the inferior regions where the convection currents in the eye lead them to settle.
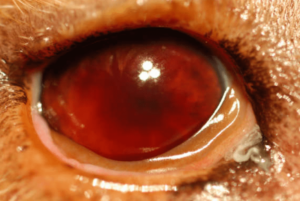
Hyphema (Photo F) is when there is a presence of blood in the anterior chamber, either as a diffuse component of the aqueous humor or as a dense precipitate accumulating in the anterior chamber (often showing a distinct red line or diffuse–3 ball if you play pool—red coloration in various shades) obscuring all or part of the iris and pupil. Blood clots with or without fibrin (Photo G) can linger in the eye even as the uveitis is virtually resolved and long after the bleeding is stopped. They often do not pose any danger to the eye in the post-inflammatory state, however, they can lead to synechiae, or adhesions of the intraocular tissue. Anterior synechiae are adhesions of the pupil to the iris and posterior synechiae are adhesions of the iris to the lens capsule. Synechia often are seen as permanent footprints of a previous inflammatory episode and can be present in eyes that no longer have any inflammation present.
(This should be differentiated from congenital adhesions due to improper ocular development—e.g. persistent pupillary membranes in the iris or adherent leukomas which are congenintal corneal scars due to incomplete separation of the cornea and iris during development). Fibrinous inflammation (Photo G) can appear in the aqueous humor during active inflammation and is another nonspecific indicator where fibrin strands become readily visible in the otherwise clear aqueous humor. Fibrin can present in trauma, infection, or any type of uveal vasculitis.
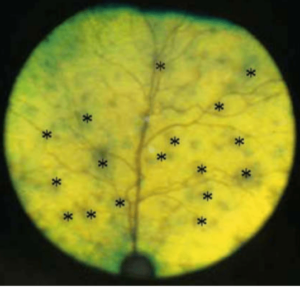
Posterior uveitis can present as an incidental finding in a routine ocular fundic examination, as a manifestation of a systemic disease work up, or as partial/total vision loss. It can be characterized as several different types of lesion descriptions, ranging from total loss of retinal attachment due to subretinal (choroidal) exudate or transudate, to a small pinpoint lesion on the fundus of one eye (Photo G). In progressive choroiditis or chorioretinitis the lesions can grow from punctate to multipunctate to coalescing changes that are observed over time.
R2: How can I predict an eye is about to break with uveitis? What are the early signs?
Subtle early changes may range from intermittent squinting, redness (conjunctival hyperemia, corneal limbal neovascularization, iridal vascular congestion/vascular dilation (Photo H), or discharge to unexplained vision abnormalities. These findings are very nonspecific and other conditions including ocular surface disease, glaucoma, cancer, etc. need to be ruled out. A patient with a recent or distant history of uveitis (e.g. from a systemic exposure to Lyme disease or a history of lymphoma) also should be scrutinized closely for relapse. Low IOP relative to the unaffected eye, aqueous flare, miosis, can all be subtle indicators of early uveitis.
R3, D1: Uveitis spiral. What are some examples of the loss of the defenses that protect the eye in uveitis?
The blood aqueous barrier breakdown is the event that is most commonly associated with uveitis. Neutralization of endogenous anti-inflammatory aqueous humor factors is another mechanism that weakens intraocular defenses (TGF-β, α-MSH, CGRP, somatostatin, etc.). Disruption of antioxidant systems mostly located in the ciliary body is another way the normal defenses of the eye are negatively affected.
R4, D2; Is vasculitis in the eye different than vasculitis elsewhere in the body?
About 98% of the blood to the eye passes through the uvea, and 85% of that blood passes through the choroid. Roughly 5% of the ocular blood flow is through the iris and 7% through the ciliary body. Blood flow through the choroid has been measured at a rate of 1400 mL/min per 100g tissue which exceeds that of the kidney. Iris blood vessels have tight junctions and lack fenestrations, rendering them in normal conditions impermeable to large molecules. Ciliary body blood vessels are fenestrated and leak much of their plasma components into the surrounding stroma as do the choroidal blood vessels. Vasculitis accentuates the preexisting permeability of the uveal blood vessels and can lead to significant disruptions in the ocular media leading to infiltration of inflammatory cells, pro-inflammatory mediators, and the potential for scarring, retinal detachment, and glaucoma.
R5, A1: What is different when uveitis coincides with another ocular disease?
Other conditions such as dry eye, glaucoma, or corneal ulceration can alter the way the concurrent uveitis is addressed. For example, atropine can dry the ocular surface and also worsen any glaucoma present, so its use would be curtailed with close monitoring, or avoided when these conditions are present. Corneal ulceration or the suspicion of any infection precludes the use of any topical anti-inflammatories without careful consideration of the ramifications of such use. A red eye must be screened for glaucoma, conjunctivitis, and dry eye even when the suspicion is high for uveitis.
R6, D3: What is phthisis bulbi and how does it happen?
Phthisis bulbi is the medical term for an end stage eye that is generally post inflammatory. The characteristics of the eye are reduced size, loss of corneal clarity often to preclude the view to the anterior chamber. Intraocular anatomy including the iris, anterior chamber, lens, pupil is often not discernable. Phthisis is the end stage to a host of conditions including intraocular trauma, glaucoma, infection, and chronic inflammation. Light perception is lost and there is no chance for vision restoration. There can be chronic ocular surface hyperemia and discharge as the globe surface loses contact with the eyelids and may develop associated inflammation from the exposure. Phthisis can be painful: it is always a good idea to ask a client if there is any rubbing or irritation of an eye that is phthisical. Chronic hypotony (low intraocular pressure) from loss of aqueous humor production is the result of long-term or severe inflammation. In humans there is a rare condition called sympathetic ophthalmia, in which inflammation from one eye (usually from trauma) leads to significant inflammation and subsequent vision loss in the other eye. There is no recognized counterpart in domestic animals per se, however, some clients who consult with Dr. Google may ask you about this condition and the chance of occurrence. This condition has been reproduced in mice experimentally, but the closest naturally occurring condition may be something like uveodermatologic syndrome as seen in northern breeds like Akitas.
R7, A2, D4: When should you throw in the towel on saving an eye from uveitis damage? (When is it ok to stop therapy when the eye is lost to vision.)
There are many considerations for electing to continue the treatment of an individual with uveitis. A few top reasons include, prognosis for return of vision is still possible (light perception is still present), client ability and compliance to medicate, side effects of medication, and most importantly, the effectiveness of the medication to treat the condition. The last reason is the one we as doctors contemplate the most but the others are not to be overlooked either.
A3: Do we always have to do a full systemic workup when we diagnose uveitis?
The best answer lies in the potential etiologies on the rule out list. If the cause for the uveitis is known trauma, then options to treat symptomatically may be appropriate. If the etiology is unknown or there is some doubt as to the cause even though you are fairly certain, then the work up is going to be an essential part of the investigation to the cause. Baseline CBC, chemistry profile, urinalysis are typically the starting points to the work up, but it can be expanded to include thoracic and abdominal imaging, lymph node aspiration, and other imaging including of the head, spinal fluid analysis, and serology testing for systemic disease depending on the species, geographic location, and the type of lesions present. Specific eye screening including ocular ultrasound and oculocentesis (anterior or vitreal chamber, subretinal aspirates) can also be part of a thorough systemic work up and is typically done by an ophthalmologist.
A4: Is topical treatment for uveitis sufficient?
For anterior uveitis, the topical treatment is often appropriate as the sole therapy. For idiopathic uveitis it is reasonable to use a combination of a topical anti-inflammatory, pain medication, and until infection is ruled out, an antibacterial drop with good intraocular penetration (e.g. ofloxacin). The frequency of treatment will be dictated by the severity of disease. We infrequently treat with antiinflammatory drops more than 6 times daily due to the risk for side effects, namely corneal epithelial toxicity and associated ulceration (discussed later). Atropine drops, if indicated, for ciliary spasm and pain can be given typically to effect dilation of the pupil, and is tapered to the lowest effective dose until the inflammation is resolved if the pain is no longer present. In severe cases, we sometimes give atropine drops even every 1-2 hours for the first 4-24 hours of treatment then taper substantially after that is there is a positive effect. In moderate cases or if hour-to hour monitoring for dilation is not possible then up to 4-6 times daily is suitable for starting treatment with a recheck in 24-48 hours and tapering after that to twice daily, once daily, three days per week, etc. Topical treatment is not as effective for posterior uveitis since the medication cannot penetrate beyond the posterior chamber.
A5: When is systemic treatment indicated for uveitis?
When we don’t know the extent of the uveitis (i.e. if it may involve the choroid, but we can’t see past the pupil), then additional testing may be needed (ocular ultrasound or careful ocular fundus examination. The rationale behind systemic therapy is two-fold: Is there posterior segment involvement, or is there contraindication/complication when solely using topical therapy in the case for anterior uveitis treatment? The classic example for the latter is when a cat with active anterior uveitis responding to anti-inflammatory steroid or NSAID drops develops a corneal ulcer (often herpetic) as a consequence of the steroid therapy. In these cases we must stop the topical antiinflammatory drop and transition to oral anti-inflammatories such as methylprednisone or prednisolone at the lowest effective frequency. If there is choroiditis or chorioretinitis, then treatment is indicated if there is notable progression of disease or the area of the affected fundus is larger enough to concern us with vision loss. Often, systemic disease brought on by hematologic spread is the culprit in bilateral or unilateral chorioretinitis, and the treatment will be directed by the results in of history and clinical work up. It is important to note that specific treatment for a pathogen of uveitis has the potential to make the uveitis worse as the kill off of the pathogen can incite more inflammation. At times the ophthalmologist can opt to neglect specific antimicrobial therapy if the pathogen in the choroid is not causing progression of disease, the disease is not adversely affecting vision (subjective), or specific treatment has worsened the inflammation or vision in the patient. A good example of this is Toxoplasmosis chorioretinitis in the cat. Other conditions of uveitis are exclusively treated with systemic medication or a combination of topical and oral medication, such as uveitis associated with hypertensive retinopathy. In these patients, the treatment targets the primary condition with supportive therapy for the inflammatory signs given dependent on the patients systemic health and the extent of the inflammation in the eye.
A6: How do I know when to stop treatment for uveitis?
It is not uncommon for us to get a referral for a uveitis relapse when treatment is stopped too soon or the frequency of medication is inadequate to treat the condition. When this happens, recovery can be impaired and prolonged which can have detrimental effects on vision and comfort. . Gradual taper over weeks is the general way we address uveitis, idiopathic or not, with weekly or biweekly rechecks in active disease until the condition has been quiet for at least two weeks. Even then we warn clients that relapse potential is unknown, even with a known etiology, since the vasculature in the eye has likely undergone some adaptation or permanent change and there is potential for immune memory and relapse. Quarterly rechecks are wise for the first year after recovery from a significant course of uveitis.
A7: How often do we need to check the eye of a uveitis patient? R8: What should a uveitis exam include?
Uveitis examination should include intraocular pressure and ulcer check, as well as a thorough inquiry to the client as to any signs of relapse or vision changes. While most cases of uveitis are unknown and pass as a one time event, the monitoring for redness, aqueous flare, pupil size, squinting, discharge, etc. is very important. Active uveitis, like other ocular diseases (corneal ulceration, glaucoma), should be monitored very closely as the conditions are often dynamic, and in our practice, not until at least two serial visits of absolute quiescence are documented at least two weeks apart, do we suggest to taper to stop the anti-inflammatory medication. Often we reduce the topical anti-inflammatory medication from twice daily to once daily to three days per week with about 10-14 days with each given frequency.
A8: What kind of help can a veterinary ophthalmologist offer to a patient with uveitis that a general practitioner cannot provide?
In general many of the routine test that are needed to monitor for uveitis can happen in general practice if one has a tonovet or tonopen, fluorescein stain, and at least a slit mode on the direct ophthalmoscope. Ophthalmologists will have equipment and experience that can allow the treatment to be fine-tuned and customized to the patient, with slit lamp biomicroscope magnification on the eye to allow better identification of early relapse, and more in depth interpretation of the results of the ophthalmic testing.
D5: What are common side effects of uveitis treatment?
Corneal changes including epithelial erosions or ulceration, scarring, and dysplasia are all possible with topical steroid or NSAID drops. In fragile or dry corneas, or those with history of ulceration or infection, judicious use of topical anti-inflammatories, or consideration of systemic anti-inflammatories should be taken in to consideration. For atropine use, the major contraindications are those patients at risk of glaucoma or dry eye. If the tear production is already severely low, then chronic of frequent topical atropine use can lead to worsening of signs the ocular surface dryness. GI stasis is also another concern for atropine use, especially in horses. Glaucoma can happen relatively quickly in uveitis patients on atropine drops that have a compromised iridocorneal angle, even after one drop, so IOP must be monitored at every uveitis recheck, especially in the early stages of treatment.
D6: Is uveitis ever treated with surgery?
Uveitis is typically considered a medical condition with few exceptions. If the inflammation is from an acute or active lens rupture (phacoclastic uveitis), urgent surgery to remove the lens may be appropriate. The removal of the lens removes the nidus for the inflammation and in theory the inflammation resolves more rapidly. Other conditions that may merit surgical or procedural intervention may include TPA injections for sterile, non-hemorrhagic fibrinous uveitis that we see sometimes, for example immediately following cataract surgery or to address complications from gonioimplant clogging. In rare cases there is a profound acute septic pyo-hemorrhagic condition, or progressive hypopyon in the eye that requires intraocular irrigation and aspiration of the ocular media to dilute out the toxic contents and get ahead of the inflammatory process, giving the eye the better chance to recover without damage to the delicate tissue from the scarring and adhesions the severe inflammation brings. These cases are typically reserved for the ophthalmologist to manage as they require intraocular surgery with special instrumentation and careful postop day to day monitoring. More complex cases of uveitis may require intraocular injections of antimicrobials or anti-inflammatories which are not surgeries per se, but often require technically difficult placement of the medication and general anesthesia for proper injection technique. Ophthalmologists will almost exclusively handle these injections. There is no surgical treatment for uveitis per se, but in some patients surgery can be adjunctive treatment to the medical regimen.
Obviously when an eye has irreversibly lost vision and light perception and is considered painful then enucleation is a humane a viable option to resolve the pain, reduce the medication burden, and also confirm the working diagnosis. Pathology should be submitted on the globe and adnexa and not discarded to the trash bin. Intrascleral and intraorbital prosthetics should not be used in uveitis cases due to the potential for complications from smoldering or relapsing inflammation and the potentially compromised ocular tunic tissue (cornea, sclera) from any bystander injury. There are cases of autoimmune uveitis that have had successful placement of intraocular prosthetics, but caution should be taken to consult with the client carefully about the potential risks involved.
D7: Why do some uveitis conditions resolve more quickly than others?
This is perhaps the hardest question to answer because so many patients with uveitis resolve without a definitive diagnosis, and in these patients the uveitis is a one-off event. The chronic relapsing cases or the recurrent cases that were previously inactive are the more challenging situations. Our call to action with regard to treatment is often to react to the symptoms observed, and that is in part due to the unpredictable nature of the inflammation in the eye. Trauma is often one of the more predictable types of inflammation we treat, and if there are no complicating factors such as lens capsule rupture or infection (such as in blunt trauma), the healing is usually gradual and stable, although the prognosis for vision more variable. The chronic cases may have a more permeable blood aqueous barrier, or persistent pathogen (antigen), autoimmunity, or memory T cells that perpetuate the condition.
D8: Can an eye recover from uveitis without treatment?
Nature’s way to recovery is through innate and adaptive immunity. The question is how the delicate tissue of the eye can manage the recovery without profound damage including glaucoma, cataract, retinal detachment, or phthisis. The immune defenses of the eye can blunt the impact of the primary immune responses, but often the symptomatic and specific medication will shorten the course of disease and preserve vision better.
D9: How do we redirect the disease to make the outcome better?
The best way to have a positive impact is to start the treatment as soon as the condition is recognized and monitor through and beyond the resolution of the inflammation. Symptomatic treatment is the first line for the inflammation, but must be used in the context of the underlying problem. For example, in rare cases the anti-inflammatories can exacerbate the uveitis by enabling a pathogen to proliferate. A good example of this is the dilemma of using systemic steroids at high doses when a patient presents for bilateral chorioretinitis due to fungal disease, or other raging infection.
D10: What factors complicate uveitis recovery?
Misdiagnosis is one. Inappropriate frequency or medicine dosing of medication is another (i.e. not often enough or not long enough or the anti-inflammatory is not strong enough). Progressive intraocular neoplasia (Photo I), primary or metastatic can be associated with a persistent uveitis that responds poorly to symptomatic treatment.
D11: What are the odds of suffering permanent vision loss from uveitis?
Vision loss from uveitis stems from various factors with caveats. Cataract, retinal detachment, corneal scarring, clouding of the aqueous or vitreal humor, and bleeding are all able to contribute to permanent vision loss. Some of these conditions can trigger inflammation (for example, cataracts, corneal disease, retinal detachment), so it can be difficult to know if the uveitis is the initial, direct cause for the vision loss, or part of a larger picture of changes in the eye that lead to vision impairment or blindness.
In summary, any condition involving inflammation inside of the eye has the potential to be complicated and can lead to varying degrees of intraocular damage and vision changes. To understand the dynamics of uveitis one would have to integrate several disciplines including the following: immunology, pharmacology, microbiology, physics, optics, and local anatomy, as well as systemic medicine in order to implement a sound strategy for treatment that leads to recovery and preservation of vision. The eye is by and large a closed space, and this offers both advantages and disadvantages to a patient with uveitis. The goals of care are to preserve vision and restore balance by eliminating the inflammatory process and anything that drives it.
Additional Reading and References:
The Eye: Basic Sciences in Practice 4th by John V Forrester, Andrew D. Dick 2016
Veterinary Ophthalmology: Two Volume by Kirk N. Gelatt, Brian C. Gilger 2013