-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
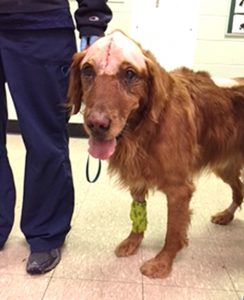
In January 2021, Angell was granted approval to participate in the Canine Immunoneurotherapeutics, or CANINE, trial. Angell’s Jennifer Michaels, DVM, DACVIM (Neurology) and Michele James, DVM, DACVIM (Neurology) are excited to work with Dr. Melissa Chambers and her team at University of Alabama – Birmingham who are leading the trial. The CANINE trial, currently underway with more than 25 dogs from across the U.S., is evaluating the effectiveness of combination immunotherapy for gliomas. The study has received funding from the National Institutes of Health to use human therapies to treat and study canine brain tumors.
After surgery for resection of the tumor—and the placement of a catheter into the tumor bed— M032, the FDA-approved, clinical-grade oncolytic herpes simplex virus that expresses an IL-12, is administered post-operatively, via a single injection. Patients are monitored overnight in the intensive care unit. They are typically discharged to home several days after surgery. Following the procedure and discharge, dogs taking part in the trial are treated for thirty days with an oral small molecule inhibitor. All dogs will be followed with regular check-ups for a full year to determine how well treatment prevents tumor regrowth and extends life. Return visits are scheduled for 1, 3, 6, 9 and 12-months for close monitoring and serial imaging.
The owners of dogs taking part in the trial are responsible for the cost of initial diagnostic testing to determine if a glioma is present, but once a diagnosis is confirmed—and pets meet all other inclusion criteria—the patient can be enrolled, with nearly all subsequent costs covered by the study. There are no placebos in the study—every participant receives active treatment.
For more information regarding this trial, please visit uab.edu/medicine/caninetrial.
To refer a patient for consideration please contact Angell’s Neurology service at 617-541-5140 or neurology@angell.org.
Please do not hesitate to reach out directly to Dr. James or Dr. Michaels with any questions:
Michele James, DVM, DACVIM (Neurology)
mjames@angell.org
617-522-7282 x5617
Jennifer Michaels, DVM, DACVIM (Neurology)
jmichaels@angell.org
617-522-7282 x5612