-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Sue Casale, DVM, DACVS
By Sue Casale, DVM, DACVS
angell.org/surgery
617-541-5048
Thoracostomy tubes or chest tubes are an important part of postoperative care following thoracic surgery. Chest tubes can be placed either pre-operatively or during surgery. Patients that have a pneumothorax or pleural effusion should have a chest tube placed prior to surgery to help with pre-operative imaging and stability for anesthesia. In all other patients, placement during surgery is safer and easier. A chest tube must be placed in any patient undergoing thoracic surgery or any abdominal surgery where the diaphragm is compromised, such as a diaphragmatic hernia. The chest tube is used for evacuation of residual fluid or air in the chest as a result of surgery and to reestablish negative pressure, allowing the lungs to expand. The chest tube is also used to drain any continued fluid or air associated with the thoracic pathology such as chyle in patients with chylothorax. Chest tubes can be used to monitor for any post-operative hemorrhage or air leakage from the surgical site as well and can be used in post-operative pain control.
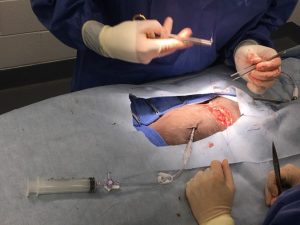
Figure 1: A chest tube must be placed in any patient undergoing thoracic surgery or any abdominal surgery where the diaphragm is compromised.
There are several types of chest tubes available to veterinarians. The most common type is the Argyle™ thoracostomy tube (see Figure 2). Argyle tubes are made of polyvinyl chloride (PVC) and come with a large sharp trocar for introducing the tube into the thorax. A second type of trocar tube is the Axiom® which is made out of silicone. Silicone is softer than PVC and therefore is thought to be more comfortable. Because Axiom trocar tubes are softer, they may be more prone to collapse when aspirating the tube. Trocar tubes are typically placed with the tube tunneled under the skin for two to three intercostal spaces before entering the chest. Alternatively, forceps can be used to guide the tube into the chest cavity rather than using the trocar. This is not recommended routinely as a study by Yoon et al. showed that trocar-implemented thoracostomy tube placement resulted in less air leakage when compared to the forceps technique. All Argyle and Axiom tubes have a radiopaque line that makes them visible on radiographs. Additional fenestrations can be added to the tubes with scissors as long as the openings that are created do not exceed 1/3 the diameter of the tube as this can result in part of the tube breaking off in the chest at removal of the chest tube.
Trocar tubes are available in a wide variety of sizes, from 8 French to 32 French. Traditionally the recommendation was to use the largest bore tube that would fit in a patient to reduce the risk of clogging. A tube the same diameter as the main stem bronchus on radiographs was recommended. This recommendation has not been supported in the human literature as occlusion and complication rates between large and small bore tubes was found to be similar. Because large bore tubes have been shown to be more painful than small bore tubes, the recommendation is to choose the tube size based on need for a particular surgical condition rather than the size of the patient.
Another popular chest tube option is the MILA™ Guidewire Inserted Chest Tube. MILA tubes are made of polyurethane and have multiple fenestrations along the tube. They are small bore, only 12 or 14 gauge. A new 12 French version has been introduced but that is still considered small for a large dog. Because of the size, use in conditions that have very thick exudate or large amounts of fibrin should be avoided. MILA tubes are placed using the Seldinger technique where an 18 gauge over-the-needle catheter is introduced into the chest and guidewire is placed into the chest through the catheter and the catheter is then removed. A dilator may be used to increase the size of the opening. The thoracostomy tube is then advanced into the chest over the guidewire and the guidewire is removed. The MILA tubes are not tunneled under the skin because of their small size but we have occasionally run in to problems with air leakage with these tubes. One advantage of MILA tubes is that they can be placed in mildly sedated animals because of their small size.
Radiographs should be taken post chest tube placement to confirm position within the chest. The tube should run along the sternum but not extend into the cranial mediastinum where the fenestrations can be occluded by tissue. All fenestrations should be within the thorax and there should be no kinks in the tube.
Once a chest tube is in place, intermittent aspiration is recommended. I will usually start with aspiration every hour for the first two hours followed by every four hours after that. If large amounts of air or fluid are being removed, I would continue with hourly aspiration until the amount decreases. If the volume is excessive or if negative pressure is not obtained, continuous suction should be used as in the case of patients with damaged lungs. Additional surgery may be necessary if a leaking staple line or bleeding is suspected. All chest tubes should have a one-way valve or an adapter with a clamp and a three-way stopcock securely attached to avoid an iatrogenic pneumothorax and to make aspiration easier. Sterile technique should be used while aspirating the chest tube and no more than 3 to 5ml of negative pressure should be applied with a syringe to avoid damaging the pleura and pulling tissue into the fenestrations in the tube.
Chest tubes may also be used to help with pain control as they can be used to introduce bupivacaine into the thoracic cavity. Conzemius et al. showed that slow infusion of 1.5mg/kg of bupivacaine into the chest tube while the patient is positioned with the incision side down, resulted in significantly lower pain scores when compared to intravenous buprenorphine.
Chest tube removal should be considered when there is little production over a 12 hour period. What defines normal production from a chest tube is debatable. The general rule is often 1-2ml/kg/day. Marques et al. reported removal of chest tubes at a mean production of 3ml/kg/day in dogs and 5ml/kg/day in cats. A recent study by Hung et al. looked at fluid production from chest tubes in normal dogs. A minimal amount of fluid was produced with the mean production at 0.48 mL/kg. A majority of dogs in that study developed a pyothorax between day 4 and 6 and the authors concluded that chest tubes should be removed by day 4 in most dogs. Removal should be based on numerous factors including patient status, disease process, fluid cytology and culture and radiographs to assess residual fluid and air.
References:
- Conzemius, MG, Brockman, DJ, King, LG, Perkowski, SZ. Analgesia in dogs after intercostal thoracotomy: a clinical trial comparing intravenous buprenorphine and interpleural bupivacaine. Vet Surg 1994; 23(4):291-298.
- Fetzer TJ, Walker JM, Bach JF Comparison of the efficacy of small and large-bore thoracostomy tubes for pleural space evacuation in canine cadavers. J Vet Emerg Crit Care 2017; 27(3):301-306.
- Hung, GC, Gaunt, MC, Rubin, JE. Quantification and characterization of pleural fluid in healthy dogs with thoracostomy tubes. Am J Vet Res 2016; 77(12):1387-1391.
- Marques, AI, Tattershall, J, Shaw DJ, et al. Retrospective analysis of the relationship between time of thoracostomy drain removal and discharge time. J Small Anim Pract. 2009; 50:162-166.
- Valtolina, C, Adamantos, S. Evaluation of small-bore wire-guided chest drains for management of pleural space disease. J Small Anim Pract 2009: 50:290-297.
- Yoon, HY, Mann, FA, Lee, S, Branson, KR Comparison of the amounts of air leakage into the thoracic cavity associated with four throacostomy tube placement techniques in canine cadavers. Am J Vet Res 2009; 70(9):1161-1167
- Yoon, HY, Kim, JS, Lee, S, Jeong, SW. Comparison of the amount of air leakage into the thoracic cavity associated with three thoracostomy tube materials in canine cadavers. Int J Appl Res Vet Med 2011; 9(4):369-376.
