-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Courtney Peck, DVM, DACVECC
By Courtney Peck, DVM, DACVECC
angell.org/emergency
MSPCA-Angell West
781-902-8400
Immune mediated hemolytic anemia (IMHA) occurs when the immune system targets and destroys the red blood cells, generally leading to acute and severe anemia. This disease commonly requires aggressive stabilization, often with multiple packed red blood cell transfusions, as well as long term management with immunosuppressive therapy and prophylactic anti-thrombotic therapy. Complications of IMHA include life-threatening anemia, hyperbilirubinemia, and thromboembolic events. Recent veterinary studies have shown the mortality rate for IMHA approaches 70%.
Historically, patients severely affected by IMHA require multiple blood transfusions to maintain hemodynamic stability until immunosuppressive therapy takes effect; this can often take up to a week. During this time, patients continue to be at risk for recurrent episodes of hypoxemia and thromboembolic events, often times requiring prolonged hospitalization until their anemia stabilizes and improves. Multiple blood transfusions increases the likelihood of transfusion reactions. Furthermore, the risk of secondary organ damage and failure due to thromboembolic events increases the longer it takes to stabilize the disease. This results in increased emotional and financial investments by their families.
More recently, therapeutic plasma exchange (TPE) has been used to successfully and rapidly gain control of the aberrant immune response in dogs suffering from severe IMHA. TPE preferentially removes biologic substances of high molecular weight such as autoantibodies, alloantibodies, endogenous toxins and antigen-antibody complexes. It can also be used to remove some exogenous drugs in cases of overdose.
There are two main techniques for TPE: continuous-flow centrifugation-based and membrane-based. Centrifugation-based TPE (cTPE) uses a specialized centrifuge to separate components of the blood based on density. Membrane-based TPE (mTPE) uses a continuous extracorporeal machine (as is used for hemodialysis), as well as a specific membrane which separates the blood components based on molecular weight. With both of these techniques, the plasma portion of the blood, which contains immunoglobulins and other protein-bound molecules, is separated from the blood and discarded. The removed portion of plasma is then replaced with a combination of donor plasma, isotonic crystalloids, and colloids.
A large bore central venous catheter (similar to those used for hemodialysis) is placed to allow rapid removal and circulation of blood through the extracorporeal machine and filter. Patients are routinely anti-coagulated during their treatments, and additional anti-thrombotic therapy is recommended between treatments as well. Each treatment aims to remove and replace one to one and a half “plasma volumes.” Plasma volume is estimated by the following formula: 0.08 x body weightkg x (1-Hematocrit), assuming blood volume is 8% of body weight. By removing 1.5 plasma volumes, TPE removes 80% of the autoantibodies in the plasma, rapidly reducing their ability to destroy red blood cells. Since the body will continue to produce autoantibodies, repeat treatments are recommended until the anemia has stabilized. The clinical consequences of IMHA (anemia, auto-agglutination) often start to improve after the first treatment. TPE treatment for IMHA usually requires two to four treatments (one treatment per day), each lasting approximately three to four hours. TPE also removes significant amounts of bilirubin, which may help reduce nausea.
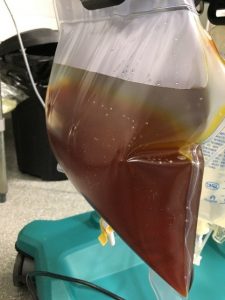
Bag of hyperbilirubinemic plasma removed from patient during a membrane-based TPE treatment that will be discarded.
In patients with IMHA, TPE removes plasma containing the autoantibodies responsible for red blood cell destruction. This allows rapid control of this disease, and can minimize the number of required packed red blood cell transfusions, risk for thromboembolic and other complications, and potentially reduce hospitalization time as compared with conventional management. Recently, Cowgill et al (ACVIM 2019 presentation, publication pending) evaluated the use of TPE for treatment of IMHA in 19 dogs. This data found that the 30-day survival rate in dogs treated with TPE was 80%, compared to a 30-day survival rate of 54% in dogs treated with traditional medical management. While the use of TPE in management of IMHA is a newer modality, the results thus far appear promising.
The use of TPE in treating IMHA does not replace the need for long term monitoring and management with immunosuppressive and anti-thrombotic therapy. However in those patients that are severely affected, TPE provides a method to rapidly gain control of the disease and improve patient outcomes. Data is not currently available on whether the use of TPE in management of IMHA affects the risk for relapse. In general, TPE should be considered when a patient with IMHA has required 2 packed red blood cell transfusions without stabilization of anemia.
Veterinary TPE also shows promise in effectively controlling other immune-mediated diseases, such as immune-mediated thrombocytopenia, fulminant myasthenia gravis, and systemic lupus erythematosus. Additionally, veterinary TPE has been successfully used in cases of severe inflammatory disease such as snake bite envenomation, hyperviscosity syndrome, and some toxicities caused by highly protein-bound compounds. While still in the early stages of clinical use and evaluation, the many potential benefits of veterinary TPE position this modality to improve our ability to provide excellent care in a variety of illnesses, including IMHA.
At Angell Animal Medical Center, we are pleased to be able to offer extracorporeal therapies including TPE to our veterinary patients. For more information, please contact either Dr. Shawn Kearns (skearns@angell.org) or Dr. Courtney Peck (cpeck@angell.org).
Selected References:
Francey T, Schweighauser A. Membrane-based therapeutic plasma exchange in dogs: Prescription, anticoagulation, and metabolic response. JVIM 2019; 33: 1635-1645.

