-
Adopt
-
Veterinary Care
Services
Client Information
- What to Expect – Angell Boston
- Client Rights and Responsibilities
- Payments / Financial Assistance
- Pharmacy
- Client Policies
- Our Doctors
- Grief Support / Counseling
- Directions and Parking
- Helpful “How-to” Pet Care
Online Payments
Referrals
- Referral Forms/Contact
- Direct Connect
- Referring Veterinarian Portal
- Clinical Articles
- Partners in Care Newsletter
CE, Internships & Alumni Info
CE Seminar Schedule
Emergency: Boston
Emergency: Waltham
Poison Control Hotline
-
Programs & Resources
- Careers
-
Donate Now
 By Elton Chan, DVM
By Elton Chan, DVM![]()
angell.org/emergency
emergency@angell.org
617-522-7282
March 2022
GDV – What Is It? “Bloat”
Gastric dilatation and volvulus (GDV) is a life-threatening surgical emergency involving inappropriate distension and displacement of the stomach, primarily affecting older, large-breed dogs. This process happens suddenly and without warning, which is very surprising for most families since these patients may not have any preexisting health concerns.
Signalment
Predisposed breeds include Great Danes, German Shepherds, Irish Setters, Standard Poodles, and Bassett Hounds.i
Modeling/Representative of GDV
Follow this link for a great 10-second YouTube video illustrating the process of GDV:
https://www.youtube.com/watch?v=JaAN-6FrPTM&ab_channel=PattersonVeterinary
As you can see from this video: The duodenum (first section of the intestine) inappropriately twists and tracks across the body wall. This prevents food/ingesta from exiting the stomach down the intestines. This also prevents ingesta from moving out orally due to twisting the esophagus, producing the classic symptom of “non-productive retching.” Dogs will typically attempt to vomit but fail to bring up gastric material due to this phenomenon.
Result of Twisting = SHOCK
The stomach twisting is followed by severe dilation since nothing can exit the chamber, and gastric contents continue metabolizing, creating more gas as a byproduct. The severe distension displaces other organs and obstructs blood from returning to the heart from the low-pressure blood vessels. This leads to a clinical manifestation of shock whereby the patient becomes weak due to a drop in blood pressure. The blood is being sequestered away from the heart due to the stomach’s distension leading to a drop in stroke volume. Additionally, blood vessels in the stomach and spleen are commonly torn during this twisting process, leading to mild internal bleeding. The sympathetic nervous system is activated to compensate, releasing adrenaline, and the heart rate increases to improve cardiac output (cardiac output = stroke volume x heart rate). This is now a life-threatening situation since the heart cannot supply adequate blood to the brain, lungs, kidneys, and other organs. Clinically, dogs begin showing signs of shock with weakness, pale gums, prolonged capillary refill time, and severely high heart rate with inappropriately low blood pressure. Many families arrive at the hospital with a presenting concern that the patient is non-productively retching, weak, unable to walk, and pale; however, it should be noted that symptoms depend on timing. Some patients present early in the process with only non-productive retching, while others arrive already deceased.
Diagnosis/Confirmation of GDV
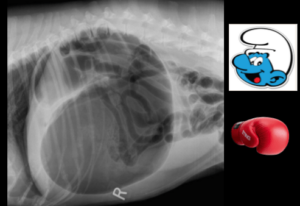
“Classic” GDVs have typical appearances described as “Smurf Hats,” “Popeye Arm,” “Boxing Gloves,” etc. to describe the compartmentalization of the stomach secondary to the inappropriate twisting and dilation.
Other disease processes can present similarly to GDV. Large, older breed dogs commonly present to the ER for sudden signs of shock for other reasons such as internal bleeding from ruptured abdominal tumors, perforated gastrointestinal tract, or heart failure from causes like dilated cardiomyopathy. Distinguishing GDV from those other processes is commonly done via abdominal radiographs. The nice thing about GDVs is that they usually have a classic appearance that helps distinguish them from other disease processes.
Diagnosis is usually achieved using a RIGHT lateral abdominal radiograph to show a distended, compartmentalized stomach. See examples of “Smurf Hat” and “Boxing Glove” for comparison. NOTE: Clinicians can sometimes be tricked by the less-common 360-degree GDV, which fails to have the classic appearance on the RIGHT-lateral projection but can be sometimes seen on the LEFT-lateral projection. I would recommend taking a full 3-view abdominal study if the RIGHT-lateral fails to confirm GDV, but the clinician retains a high level of suspicion of a GDV. (Below, an example of 360-degree GDV caught on LEFT lateral projection instead of standard RIGHT-lateral.) Additional radiographic features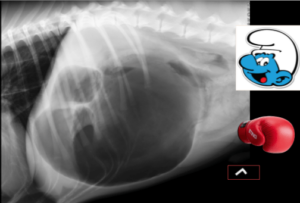 common to patients with GDVs include loss of serosal detail, gas tracking along the wall of the stomach, dilated esophagus, and diffusely gas-distended small intestines. These are additional pathologic changes associated with the inappropriate twisting and distension of the stomach, which suggest temporary paralysis of the intestines (functional ileus), internal bleeding, and tearing/necrosis of the stomach mucosa.
common to patients with GDVs include loss of serosal detail, gas tracking along the wall of the stomach, dilated esophagus, and diffusely gas-distended small intestines. These are additional pathologic changes associated with the inappropriate twisting and distension of the stomach, which suggest temporary paralysis of the intestines (functional ileus), internal bleeding, and tearing/necrosis of the stomach mucosa.
Chest Radiographs
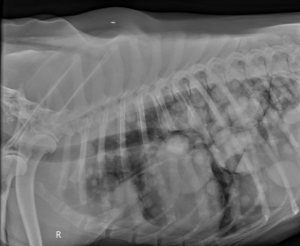
Chest radiographs of a dog with numerous metastatic lung tumors
Performing chest radiographs is always recommended for these patients since this disease process is highly concentrated in older patients. Chest radiographs are essential to ensure that significant comorbidities do not exist (such as metastatic cancer) or pneumonia which may significantly worsen the patient’s prognosis. I would not recommend taking a patient in for emergency surgery if there are signs of metastatic cancer since the prognosis is poor, and the patient may be at increased risk of anesthetic death if there is significant lung disease. It should also be noted that some studies have shown an incidence rate of pneumonia as high as 14% in patients affected by GDVs, and this is important to know before going into surgery and for post-op management.ii
Our favorite body harness is called the Freedom No-Pull Harness (pictured below), which comes with a unique leash so you can hook the leash to the front and top rings. If you “pay” your dog a treat at your left side and feed them every two to four steps, they will hang out at your left side more often.
The Role of Bloodwork
The mainstay of diagnosis is imaging; however, bloodwork can help provide some useful information. Lab work results are highly variable in these cases and can indicate additional complications such as anemia (from blood loss) and kidney/liver injury from shock. These findings are not specific to GDVs and are common to other causes of shock, such as bleeding intraabdominal tumors. One piece of lab work that has some benefit is lactate which can be obtained from venous blood gas. Lactate is a molecule produced during anaerobic metabolism, which commonly occurs during shock. iii, iv, v Earlier papers showed that dogs with lactate concentrations higher than 6 mmol/L are associated with a higher incidence of gastric necrosis and worse outcome. Later papers argued that using a cutoff of 6 mmol/L was insufficient as a predictor and that it is important to gauge a patient’s response to fluid resuscitation as a better predictor of outcome. A recent paper shows that a more appropriate cutoff for worse outcome was 7.4 mmol/L. The major takeaway from this is that you cannot diagnose a GDV from bloodwork and that some lab values such as lactate have been used to try and gauge the risk of complications and outcome, and the higher a number is, the more worried we generally are; however, the number itself is not a clear indicator on prognosis, and overall prognosis for this disease process is fair-to-good with survival reported as high as 90% in some studies.vi As a referring veterinarian, it makes sense to me that I would feel more concerned about the patient with a lactate of 9 mmol/L compared to a dog with a lactate of 3 mmol/L. I might feel inclined to refer that patient with a higher lactate to a specialty hospital instead of cutting it myself if I am not confident in my abilities to perform the surgery or provide intensive post-operative care.
Treatment Part 1 – Stabilization
Stabilization commonly occurs concurrently with diagnostic workup since these patients usually arrive in a state of shock. It is fairly common to have patients triaged to the critical care unit for intravenous catheter placement based on physical exam findings of shock/weakness while the definitive diagnosis has yet to be achieved. In recognizing that cardiac output equals stroke volume multiplied by heart rate, stabilization commonly involves a large initial dose of intravenous fluids to immediately address deficits in stroke volume caused by the stomach’s distension. Once a diagnosis has been achieved (typically via abdominal radiographs), the next step of stabilization involves deflating the stomach to reduce obstructive pressure preventing blood from returning to the heart that has been impairing normal stroke volume. Gastric decompression can usually be achieved via trocharization (taking a long needle and physically stabbing the stomach through the body wall) or passing an orogastric tube (manually passing a tube down the mouth and esophagus into the stomach to pump out gastric contents). Standard of care and experience will dictate which method is pursued, but the goal and outcome are generally the same: deflate the stomach to allow blood to return to the heart, improving stroke volume. There are pros and cons to both methods. Trocharization (stabbing the stomach with a needle) is relatively easy and minimally invasive to deflate the stomach, which doesn’t take much training. The downside is that the needles are relatively narrow in diameter and are not as effective in removing gas and fluid from the stomach. There is also a low risk of lacerating the spleen during the insertion of the needle. Passing an orogastric tube can relieve more stomach contents; however, it typically requires significantly more sedation since it requires forcing a large-diameter plastic tube down a dog’s throat. It also has the risk of causing aspiration pneumonia (if stomach contents leak around the tube and go into the lungs) and perforation of the esophagus.
Some clinicians trocharize the stomach and then pass a smaller, less invasive nasogastric tube instead of an orogastric tube. Hospital standards of care and training dictate which methodology is performed. This part of stabilization DOES NOT fix the twisting of the stomach. It just relieves the pressure to stabilize the patient’s cardiovascular status. It buys time. Definitive therapy requires abdominal surgery to UNFLIP the stomach and surgically anchor it to the body wall to prevent the stomach from rotating again.
Making the Call – Proceed vs. Stopping Point (Euthanasia)
At this point of the workup, we should have achieved diagnosis and some degree of stabilization so that the patient is not at immediate risk of dying on the triage table. It is now time to make the call on whether to move forward with surgery or consider humane euthanasia once the family has reached a better understanding of the severity of the disease process and why their pet suddenly became sick without warning. The family should be guided that this disease process typically happens in older large breed dogs but can be surgically corrected. Outcome and prognosis are generally fair-to-good, with some survival and post-op success reports reaching 90%. Patients are typically discharged, walk out of the hospital one to three days following surgery, and then live their normal quality of life without significant life-long implications. This is not an inexpensive emergency investment in what is typically a geriatric patient, so each family should weigh the pros and cons of making this investment based on their specific life situation. In 2022, I typically give patients a cost estimate of $6,000 to $8,000 for patients diagnosed and treated with GDVs. Costs can vary widely due to the risk of complications, such as aspiration pneumonia and prolonged hospitalization, and for some families, this is a stopping point that is not unreasonable.
Treatment Part 2 – Surgical De-rotation and Gastropexy
Assuming that the family has elected to continue therapy, the next step is definitive surgery to de-rotate the stomach and anchor it to the body wall to prevent a recurrence. A wide mid-line incision is typically performed to provide the best visibility and access to the abdominal cavity. The stomach is de-rotated and assessed for viability. It is not uncommon for sections of the stomach to be injured due to strangulation secondary to its twisting. Some of these areas are salvageable once de-rotation is achieved and blood supply has been restored to the stomach; however, some areas may have died off and need removal since they represent a structural liability for perforation and infection. Surgeons typically ask the nurse to pass an orogastric tube at this point once the stomach is de-rotated and the patient is anesthetized with the airway protected with the endotracheal (anesthesia) tube to minimize the risk of aspiration pneumonia. The stomach contents are pumped out via the tube into a bucket. The surgeon typically assesses the rest of the abdominal cavity to ensure nothing else is concerning. It is not uncommon to remove the spleen since it is often injured secondary to twisting the stomach with its blood vessels avulsed. There are no long-term concerns about this removal of the spleen. The surgeon then anchors the stomach to the body wall using a suture to prevent recurrence. This anchoring process is called a “gastropexy.” At this point, the surgery is finished, and the abdomen is closed.
Post-Op Care
Just because the patient is out of the operating room does not mean that it’s out of the woods. Post-op GDV patients commonly produce abnormal heart rhythms secondary to shock and reperfusion injury following fluid resuscitation and de-rotation of the stomach. Patients are commonly hospitalized post-op with continuous ECG monitoring to evaluate the heart’s electrical activity and prompt intervention. The most common aberrant electrical pattern these patients exhibit is an Accelerated Idioventricular Rhythm involving aberrant premature ventricular complexes that can evolve into a life-threatening situation. Intravenous lidocaine is typically used to manage this, but additional drugs such as Sotalol can be considered, and the rhythm usually resolves within a few days post-operatively since the source of shock and myocardial injury has been addressed. Patients are typically sent home in 1-3 days post-op so long as they remain hemodynamically stable (normal heart rate, blood pressure, vitals), eating (ideally), and comfortable (minimal nausea so that they can continue taking pain-relief medications at home and not vomit them up).
Prevention
GDVs are preventable via elective gastropexy. This essentially means that instead of waiting for an emergency to happen six to seven years later, a family elects to bring a young pet of a predisposed breed to a veterinarian to anchor the stomach to the body wall surgically. This is commonly done in puppies during spay/ovariectomy in females, but it can also be done electively for male dogs as an isolated procedure. Depending on training, some veterinarians can also perform these procedures minimally-invasively via laparoscopy, making small porthole incisions without large midline abdominal incisions. Both methodologies are acceptable so long as the stomach is appropriately anchored to the body wall to prevent it from flipping years later. NOTE: We commonly see patients undergoing abdominal exploratory surgeries for other reasons, such as routine surgical retrieval for obstructive intestinal foreign bodies (like tennis balls). I would recommend offering an elective gastropexy at this time since the pet is already undergoing abdominal surgery. (It would be a shame if the pet developed a GDV years later, which could have been prevented.) Lastly, an elective gastropexy should be offered to patients based on the risk of developing GDVs, such as predisposed breeds like Great Danes and German Shepherds.
Closing Remarks
I hope this article helps you understand this disease process and sets a basic expectation on a “routine” workflow in diagnosing and treating this disease process. There is so much more to be studied about this process, including additional risk factors for GDVs (e.g., elevated food bowls, speed of food consumption, previous abdominal surgeries) that scientists are still working on answering. We know that certain breeds and age groups are at increased risk of developing GDVs but what triggers the stomach to twist in these patients remains unclear. That being said, I hope that this article helps clarify the rationale for what your ER doctor is probably thinking and doing and what your pet is going through.
References
i Glickman LT, Glickman NW, Schellenberg DB, Raghavan M, Lee TL. Incidence of and breed-related risk factors for gastric dilatation-volvulus in dogs. J Am Vet Med Assoc. 2000 Jan 1;216(1):40-5. doi: 10.2460/javma.2000.216.40. PMID: 10638316.
ii Green JL, Cimino Brown D, Agnello KA. Preoperative thoracic radiographic findings in dogs presenting for gastric dilatation-volvulus (2000-2010): 101 cases. J Vet Emerg Crit Care (San Antonio). 2012 Oct;22(5):595-600. doi: 10.1111/j.1476-4431.2012.00802.x. PMID: 23110573.
iii Mooney E, Raw C, Hughes D. Plasma lactate concentration as a prognostic biomarker in dogs with gastric dilation and volvulus. Top Companion Anim Med. 2014 Sep;29(3):71-6. doi: 10.1053/j.tcam.2014.09.005. Epub 2014 Sep 18. PMID: 25496924.
iv de Papp E, Drobatz KJ, Hughes D. Plasma lactate concentration as a predictor of gastric necrosis and survival among dogs with gastric dilatation-volvulus: 102 cases (1995-1998). J Am Vet Med Assoc. 1999 Jul 1;215(1):49-52. PMID: 10397065.
v Beer KA, Syring RS, Drobatz KJ. Evaluation of plasma lactate concentration and base excess at the time of hospital admission as predictors of gastric necrosis and outcome and correlation between those variables in dogs with gastric dilatation-volvulus: 78 cases (2004-2009). J Am Vet Med Assoc. 2013 Jan 1;242(1):54-8. doi: 10.2460/javma.242.1.54. PMID: 23234282.
vi Song KK, Goldsmid SE, Lee J, Simpson DJ. Retrospective analysis of 736 cases of canine gastric dilatation volvulus. Aust Vet J. 2020 Jun;98(6):232-238. doi: 10.1111/avj.12942. Epub 2020 Apr 6. PMID: 32253749.